Palladium nanostructures in the shape of Christmas-tree had created using a simple electrodeposition approach on a Glassy Carbon Electrode (GCE). In alkaline conditions, the modified electrode showed eight times higher catalytic activity than the unmodified GCE for Ascorbic Acid (AA) electro-oxidation. The multiple sharp edges of the hierarchical nanostructures are account for this excellent efficiency.
Nanostructured metal surface has novel physical and chemical properties, which have sparked scientific interest for heterogeneous catalysis, biosensors, and electrocatalysis. The fabrication process can influence the shapes and sizes of metal nanostructures. Among various fabrication processes, the electrochemical deposition technique is widely used for clean metal nanostructures. Applying the technique, a team of researchers led by Dr. Yuki Nagao, Associate Professor at Japan Advanced Institute of Science and Technology (JAIST) and Md. Mahmudul Hasan, a PhD student at JAIST, succeeded to construct Pd-based catalysts having unique morphology.
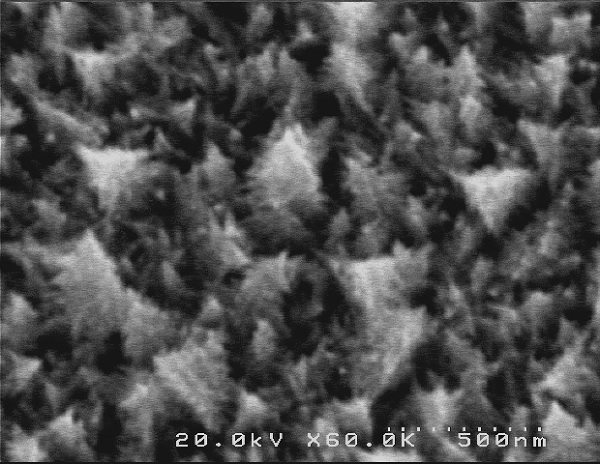
Figure 1: Unique Christmas-tree-shaped palladium nanostructures.
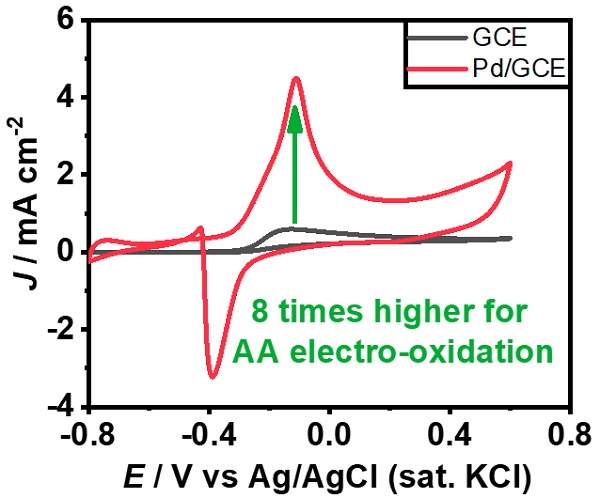
Figure 2: AA electro-oxidation is enhanced by unique metal morphology.
In this study, the team has successfully synthesized Christmas-tree-shaped palladium nanostructures on the GCE surface for the first time by one-pot electrodeposition without using any additives (Figure 1). The controlled electrodeposition method creates many sharp edges of Christmas-tree-shaped palladium nanostructures (Pd/GCE) that enhanced the catalytic activity for AA electro-oxidation.
The unique nanostructures on the GCE exhibit excellent electrocatalytic oxidation of AA than the unmodified GCE in 1 M KOH solution (Figure 2). Multiple sharp edges observed in the nanostructures improved the electrocatalytic performance. This brings one step closer to the construction of alkaline AA-based Direct liquid fuel cell (DLFC.) “Improving the electrocatalytic performance of AA electro-oxidation could provide cleaner energy by constructing alkaline AA-based DLFC.” explains Hasan.
To address the energy crises and climate change, clean energy sources need to be explored urgently. DLFC could be a potential candidate for the new energy source with its simple cell design. AA, known as vitamin C, is a feasible fuel source for DLFC. AA is environment-friendly and generates two electrons and two protons along with green dehydroascorbic acid during its electro-oxidation. AA is more affordable and, thus, can be used as a clean energy source widely.
This research could mark a firm step forward in achieving sustainable development goals.
Read the original article on AlphaGalileo.
