The combination of organic and inorganic components in hybrid nanomaterials can be tailored to capture, detect, convert or control light in unique ways. Interest in these materials is high, and the pace of scientific publication about them has grown more than tenfold over the past 20 years. For example, they could potentially improve the efficiency of solar power systems by harvesting energy from wavelengths of sunlight — like infrared — that are missed by traditional photovoltaic solar panels.
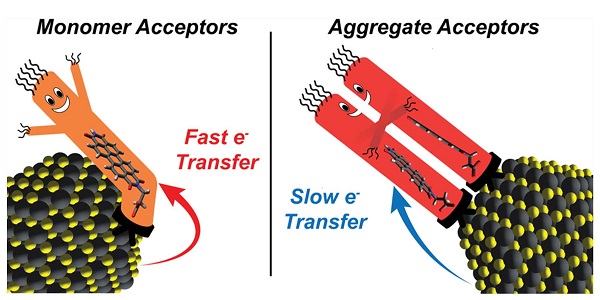
Chemists from Rice University and the University of Texas at Austin showed that adding more charge-accepting ligands to the surface of semiconducting nanocrystals can produce ligand-ligand interactions that reduce the rate of electron transfer in hybrid nanomaterials.
To create the materials, chemists marry nanocrystals of light-capturing semiconductors with “charge acceptor” molecules that act as ligands, attaching to the semiconductor’s surface and transporting electrons away from the nanocrystals.
“The most-studied nanocrystal systems feature high concentrations of charge acceptors that are bound directly to the semiconducting crystals,” said Rice chemist Peter Rossky, co-corresponding author of a recent study in the Journal of the American Chemical Society.
“Generally, people try to maximize the surface concentration of charge acceptors because they expect the rate of electron transfer to continuously increase with surface-acceptor concentration.”
A few published experiments had shown electron transfer rates initially increase with surface-acceptor concentration and then fall if surface concentrations continue to rise. Rossky and co-corresponding author Sean Roberts , an associate professor of chemistry at UT Austin, knew molecular orbitals of ligands could interact in ways that might influence charge transfer, and they expected there was a point at which packing more ligands onto a crystal’s surface would give rise to such interactions.
Rossky and Roberts are co-principal investigators with the Rice-based Center for Adapting Flaws into Features (CAFF), a multiuniversity program backed by the National Science Foundation (NSF) that seeks to exploit microscopic chemical defects in materials to make innovative catalysts, coatings and electronics.
To test their idea, Rossky, Roberts and colleagues at CAFF systematically studied hybrid materials containing lead sulfide nanocrystals and varying concentrations of an oft-studied organic dye called perylene diimide (PDI). The experiments showed that continually increasing the concentration of PDI on the surface of nanocrystals eventually produced a precipitous drop in electron transfer rates.
Rossky said the key to the behavior was the effect that ligand-ligand interactions between PDI molecules have on the geometries of PDI aggregates on crystal surfaces. Compiling evidence to show the impact of these aggregation effects required expertise from each research group and a careful combination of spectroscopic experiments, electronic structure calculations and molecular dynamics simulations.
Roberts said, “Our results demonstrate the importance of considering ligand-ligand interactions when designing light-activated hybrid nanocrystal materials for charge separation. We showed ligand aggregation can definitely slow electron transfer in some circumstances. But intriguingly, our computational models predict ligand aggregation can also speed electron transfer in other circumstances.”
Read the original article on Rice University.