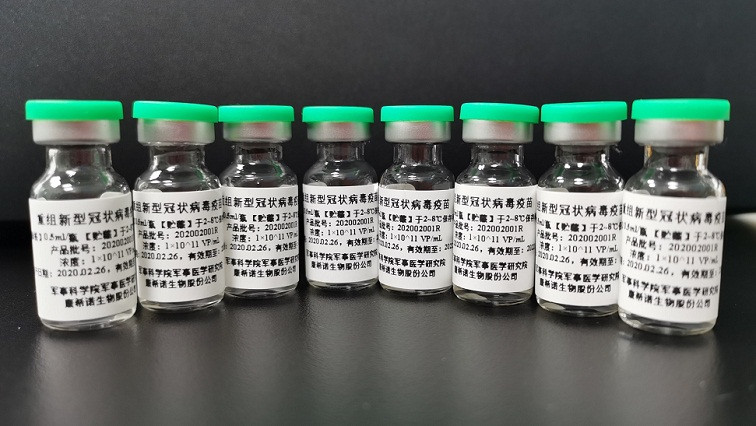
One day after the U.S. began the first human trial of an mRNA vaccine candidate for COVID-19 on March 16, China said Tuesday evening that it had approved the first clinical trial of a vaccine candidate developed by domestic researchers.
The vaccine candidate, known as Ad5-nCoV, is a recombinant novel coronavirus vaccine. It was jointly developed by Tianjin-based Cansino Biologics Inc. and the Institute of Biotechnology of the Academy of Military Medical Sciences. The clinical trial will enroll 108 subjects and take place at Tongji Hospital in Wuhan, the epicenter of COVID-19.
Cansino said Ad5-nCoV is a genetically engineered vaccine candidate with the replication-defective adenovirus type 5 as the vector to express SARS-CoV-2 spike protein. The vaccine candidate is intended to be used to prevent the disease caused by the novel coronavirus infection.
Backed by the state, the company has also obtained support from the Tianjin Science and Technology Bureau through the Project on Emergency Prevention and Control of COVID-19 Infection to develop the vaccine.
On March 17, the company said it has started to prepare for clinical trials of Ad5-nCoV and the pre-screening of healthy volunteers.
Cansino CEO Xuefeng Yu said the company and the academy have been collaborating closely since late January to develop Ad5-nCoV and generate sound scientific data to support its IND filing. “Having committed to providing unconditional support to fight against the global epidemic, Cansino is determined to launch our vaccine product candidate as soon as possible, with no compromises in quality and safety,” he added.
The primary indicator is to see whether there are adverse reactions seven days after injection, and the secondary indicators are whether adverse reactions are observed 28 days after injection or severe adverse reactions six months after the injection.
Researchers will also look for anti-S antibody immunoglobulin G, neutralizing antibodies against SARS-CoV-2, neutralizing antibodies against Ad5 and specific T-cell responses as secondary indicators.
Ad5-nCoV is developed with Cansino’s adenovirus-based viral vector vaccine technology platform, which utilizes adenoviruses as viral vectors to deliver vaccine antigens to the human cell. Previously, the technology platform was key in enabling Cansino to translate its Ebola virus disease vaccine, Ad5-EBOV, from a concept to an approved product in merely three years.
A forerunner in China’s vaccine space, Cansino came to the industry’s attention when its Ad5-EBOV became the first approved Ebola virus vaccine in China for emergency use and as part of the national stockpile in October 2017.
Leveraging the existing technology platform, Cansino said results from preclinical animal studies of Ad5-nCoV show that the vaccine candidate can induce strong immune responses in animal models. The preclinical animal safety studies demonstrated a good safety profile.
“We have completed the necessary preclinical steps for vaccine R&D. To date, the GMP clinical batches have passed quality testing and are ready for the phase I clinical trial,” the company said.
A cautious scientist, Yu stressed the importance of safety in vaccine development and warned that there is more to think of than just the speed of development.
“We need speed, but we still need quality. We don't want to introduce a second harm to people. It is critically important to check every step,” Yu said in a webinar organized by Chinese CRO Wuxi Apptec last month.
He said although the existing technology platform can speed up the development of a vaccine, there are issues to address before applying any vaccine to a human. Animal models should be available before moving any vaccine candidates into human trials so as to produce a vaccine that will eventually work without concerns such as disease enhancement.
“Even though we have learned from MERS and SARS, COVID-19 is still a new virus that behaves very differently, so we should really get some basic understanding,” Yu said.
And in developing a vaccine for emergency response, Yu said it would be ideal to require just one dose, and developers need to consider the manufacturability of the vaccine in order to benefit more people.
On March 16, the NIH said a phase I trial has begun in Seattle. The vaccine candidate, known as mRNA-1273 and co-developed by NIH and Moderna Inc., encodes viral spike (S) protein for prevention of COVID-19.
Read the original article on BioWorld.