For preparing nanostructures, top-down approaches such as lithography have since long been common. This has proven to be quite useful in semiconductor fabrication, but for more dedicated applications it is rather time-consuming and not particularly cost-effective. As an alternative, many researchers explore the bottom-up synthesis of nanostructures, for instance based on the self-assembly of molecules or nanoscale building blocks. However, achieving geometry control here often requires costly additives and surfactants, rendering large-scale material preparation quite challenging.
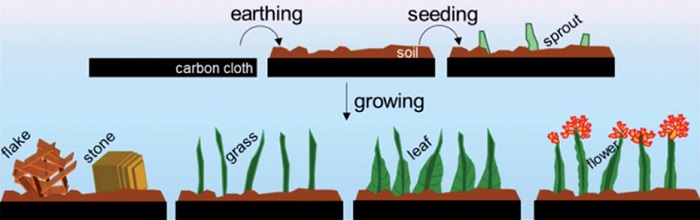
As an alternative, assistant professor Ning Yan together with his PhD students Jasper Biemolt and Pieter Laan of the University of Amsterdam's Van 't Hoff Institute for Molecular Sciences (HIMS) have now explored the relatively straightforward method of electro-deposition of cobalt hydroxide. In cooperation with researchers at the School of Physics and Technology at Wuhan University, China, they have been able to design and prepare a variety of nano-architectures that resemble various items in a garden: soil, sprouts, grasses, flowers and leaves.
In a 'hot paper' featured on the front cover of the Journal of Materials Chemistry A, the researchers report how they have mastered the system in such a way that they are able to 'grow' any of these structures at wish.
Adding to this, they were able to render the nanostructures catalytically active by a simple phosphidation procedure. The resulting cobalt phosphide nanostructures display bifunctional catalytic activity in electrolytic water splitting, enhancing both hydrogen and oxygen generation reactions.
Hierarchical nanostructures through controlled synthesis
Ning Yan and co-workers grew their nanogardens on a common electrode material in the fuel cell and electrolyser industry: a cloth consisting of carbon fibres of around 10 micrometres in diameter. The gardening started with depositing a layer of 'soil' by hydrothermally encapsulating the fibres with a dense layer of cobalt hydroxide.
This layer increased the structural stability of the nanostructures. Through variation of the ion concentration and temperature, they were able to induce the 'sprouting' of grass-like features that are strongly 'rooted' in the soil. These grasses have an average length of 1.5 mm and a thickness of around 100 nm. To add blossoms and leaves to the grassy features, the researchers applied an electrodeposition method.
In a diluted solution, electrodeposition dominantly proceeds from the tip of the grass stem, where the small radius of curvature results in a higher space charge density. In more concentrated solutions, the electrodeposition mainly proceeds from the bottom of the stems. This results in the deposition of 'leafy' features, which in fact are interwoven dendritic deposit structures.
After converting the cobalt hydroxide nanostructures to cobalt phosphide by means of phosphidation, the researchers evaluated their catalytic activity in a setting that adequately represented industrially relevant conditions. As it turned out, the performance of the catalyst in an acidic environment is among the best of today's superior non-precious metal catalysts for hydrogen evolution.
Furthermore, in acidic as well as alkaline and neutral conditions, the flowery nanofeatures resulted in significantly larger turnover frequencies than the leafy features, particularly at higher overpotentials when hydrogen evolution is influenced by mass transport limitations. The researchers attribute this to the geometry of the nanofeatures where the flowers enable smoother unloading of hydrogen. However, the different reaction environments at the top and bottom positions of the nanostructures complement each other, resulting in optimum overall performance.
Finally, in electrolysis experiments on water splitting, the researchers showed that their nanogardens not only catalyse the hydrogen evolution reaction but also the oxygen evolution. This bifunctional activity was shown using a symmetric two-electrode setup with completely identical nanogardens at the anode and cathode. The team will further investigate the use of electrons to control the growth of nanocrystals in an 'electrified' materials synthesis that holds promise for a sustainable future.
Read the original article on Hoff Institute for Molecular Sciences (HIMS).