Alloying is a general and efficient strategy to boost the catalytic activity of Pt catalysts toward oxygen reduction reaction (ORR) through electronic and geometric effects. Besides, high-index surfaces (HISs) of Pt also exhibit superior ORR activity, mainly originated from low-coordinated step or kink atoms. Thus, a combination of the alloying and HISs would be a promising method to further develop excellent catalysts for ORR. However, simultaneous control of alloy composition and HISs exposure in nanoscale remains challenging.
Recently, a research team led by Prof. Shengli Chen from Wuhan University, China designed a nanodendrite Pt-Cu alloy electrocatalyst possessing rich spiny branches exposing n(111)x(110) HISs with a graded composition of PtCu3@Pt3Cu@Pt. The electrocatalyst was obtained through an atmosphere-modulated solution-phase synthesis followed by electrochemical dealloying. The results were published in Chinese Journal of Catalysis.
Nanodendrite morphology of PtCu3 alloy is achieved through controlling the reaction atmospheres, more specifically, by initially applying an oxidative atmosphere to form concaved nanocubes of Pt-Cu seeds, and then switching to an inert atmosphere under which an explosive growth of dendrites takes place. When keeping the oxidative atmosphere, the Pt-Cu concave cubes continue to grow. If initially applying for an inert atmosphere, five-fold twins of Pt-Cu crystals form to minimize the surface energy. The five-fold twins further grow to nano-polypods under the inert atmosphere, but transform to concave cubes if switching to an oxidizing atmosphere because of the dislocations in the five-fold twins. The PtCu3 nanodendrites are surrounded by a high-index surface with a large number of steps, with the (111) planes exhibiting a lattice fringe spacing of 0.214 nm, which corresponds to a 5.3% lattice shrinkage as compared with the 0.226 nm of Pt(111).
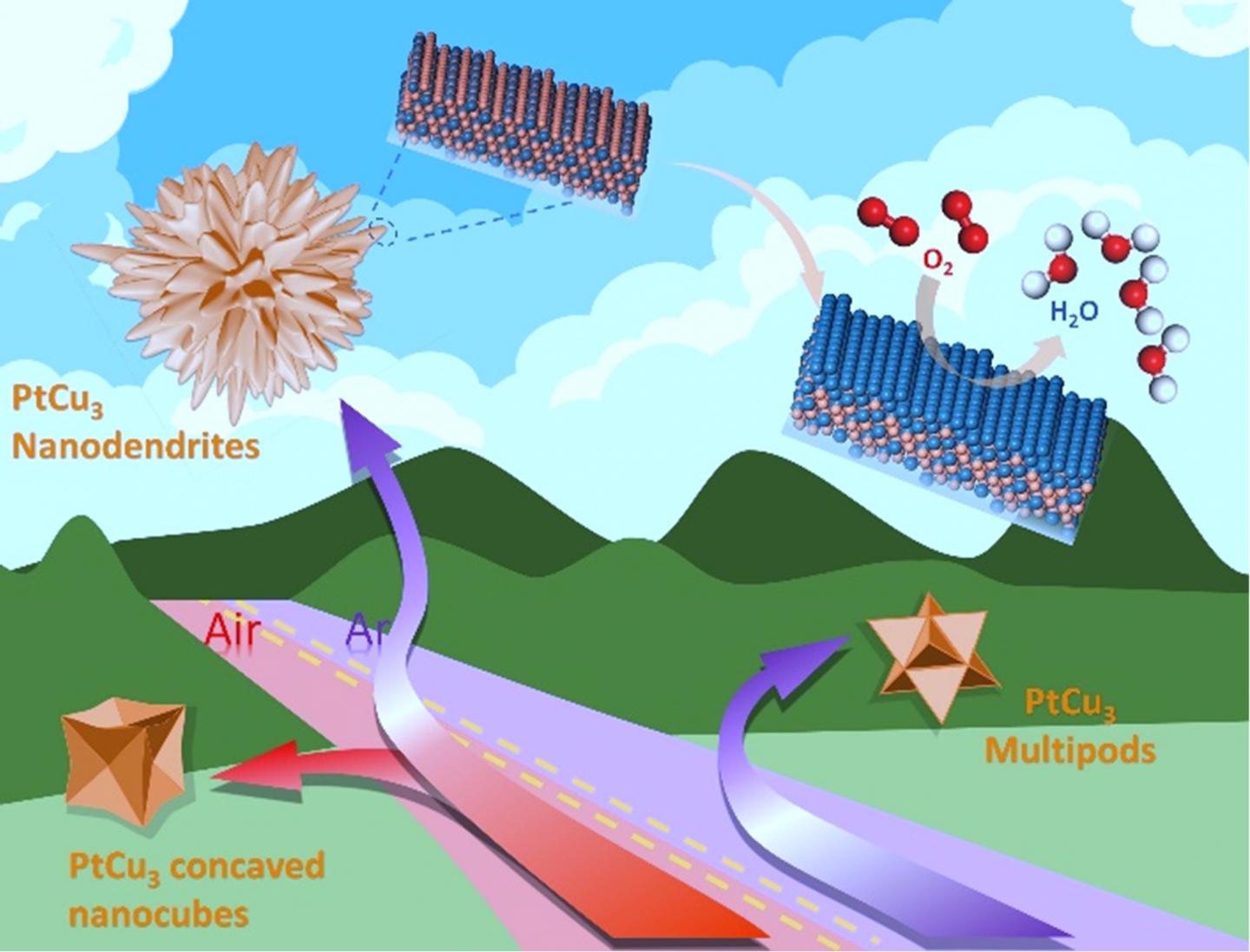
Using an atmosphere switching strategy followed by electrochemical dealloying, researchers have synthesized composition-graded PtCu3@Pt3Cu@Pt nanodendrites exposing high index surfaces, which exhibited excellent ORR activity and stability in acidic electrolytes.
Electrochemical dealloying, performed through 100 cycles of cyclic voltammogram (CV) with a scan rate of 500 mV s-1 between 0.06 ~1.3 V (vs. RHE) in O2-saturated 0.1 M HClO4 solution, was used to obtain HIS catalysts with gradient composition from the as-prepared Pt-Cu nanocrystals. A Pt-rich surface was obtained while the HISs is retained, leading to composition-graded PtCu3@Pt3Cu@Pt nanodendrites.
The nanodendritic structure and low Pt content together provide a high specific ECSA to improve the Pt utilization, and the HISs and gradient composition of catalysts together provide a high oxygen reduction catalytic activity. PtCu3@Pt3Cu@Pt nanodendrites exhibit excellent mass and area activities of Pt for ORR in 0.1 M HClO4 solution, which are 15 and 24 times higher than that of Pt/C, respectively. DFT calculations reveal that Cu alloying and HISs both have contributed to the significantly enhanced activity of Pt, and that the oxygen binding energy on the step sites of HISs on the PtCu3@Pt3Cu@Pt nanodendrites approaches the optimal value to give ORR activity near the so-called volcano top.
Read the original article on EurekAlert.