Ultra-deep removal of H2S is important in petroleum refining, natural gas purification and coal chemical industry. However, the industrial catalysts for continuous H2S selective oxidation show poor activity and stability, especially on feed gas containing steam and impurity gas.
Recently, Assoc. Prof. LIU Yuefeng's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) fabricated monolithic nanocarbon composites for continuous removal of high concentration of H2S, presenting superior product selectivity and stability under high concentration of O2, CO2 and steam.
This work was published in ACS Catalysis on June 30.
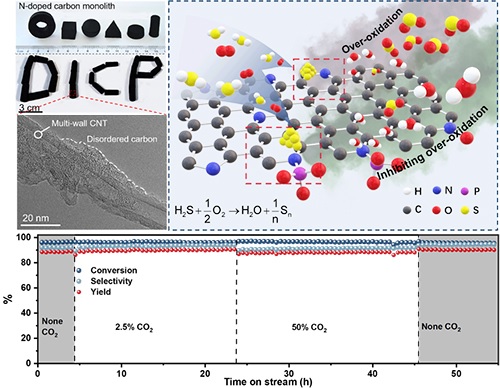
The monolithic nanocarbon catalyst presents superior catalytic performance for H2S selective oxidation with improved sulfur selectivity and impurity tolerance.
Nanocarbon materials possess unique surface chemical properties and excellent catalytic performance. However, the overactive sites and the exothermic characteristics of the reaction can cause overoxidation of product into SOX.
The researchers achieved high selectivity of sulfur for the selective oxidation of H2S without losing conversion by phosphate-modified N-doped 3D mesoporous monolithic carbocatalysts (N-C/CNT), leading to a high sulfur formation rate.
The P-modified N-C/CNT monolith exhibited high stability even under severe reaction environments with CO2, O2, steam and SO2, indicating the promising potential for practical application.
Combining advanced characterization methods (XPS, TPD), kinetic analysis and theoretical calculation, the researchers found that the interaction between the P group and pyridine site, which was the active center, could moderate the adsorption and activity of O2 on the active site, thus avoiding the occurrence of over oxidation and improving the selectivity of the product.
Read the original article on Chinese Academy of Sciences (CAS).
