The goal is to more accurately spot vulnerable plaque, or the problem areas lurking within arteries that lead to clots, and in turn heart attacks and strokes. A description of the technology was recently published in Advanced Functional Materials.
“I believe we are now closer to developing a more precise method to diagnose and treat life-threatening atherosclerotic plaques,” Ghosn says. “Our method could be deployed in combination with IVUS to significantly improve its accuracy and sensitivity, or it could be used non-invasively.”
Earlier this year, the FDA approved a photoacoustic imaging system for detection of breast cancer. Several companies are developing photoacoustic imaging systems, and what we might call “plain vanilla” PAI is currently being tested on carotid artery plaque in clinical studies in Europe.
Ghosn’s approach, developed with biomedical engineer Bryan Smith at Michigan State, adds specificity by adding nanoparticle probes taken up by macrophages, the immune cells that accumulate within atherosclerotic plaques. The nanoparticles, administered before imaging, act as contrast agents.
From science fiction movies, we might think lasers come with a “pow” sound. Photoacoustic imaging is more like listening for a whisper: sounds associated with heat generated by a laser pulse when it is absorbed by tissue.
Earlier this year, the FDA approved a photoacoustic imaging system for detection of breast cancer. Several companies are developing photoacoustic imaging systems, and what we might call “plain vanilla” PAI is currently being tested on carotid artery plaque in clinical studies in Europe.
Ghosn’s approach, developed with biomedical engineer Bryan Smith at Michigan State, adds specificity by adding nanoparticle probes taken up by macrophages, the immune cells that accumulate within atherosclerotic plaques. The nanoparticles, administered before imaging, act as contrast agents.
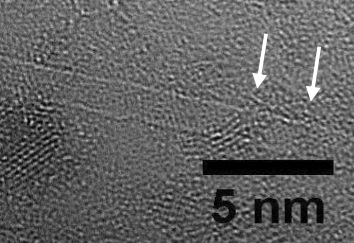
Carbon nanotubes, viewed via electron microscope.
“Our method is really looking at the monocytes and macrophages,” Smith says. “Virtually no other cell type takes up the nanoparticles.”
The particles absorb laser light and emit sound waves, which are used to locate and visualize the plaque.
“Although there are other approaches that aim to locate and treat plaques, our method specifically targets the most important cell type that contributes to disease progression,” Ghosn says.
Ghosn and Smith’s collaboration began at Stanford several years ago, with the observation that carbon nanotubes are specifically taken up by a subset of macrophages. These are the same cells that accumulate in atherosclerotic plaques.
The Advanced Functional Materials paper includes ex vivo photoacoustic imaging of mouse carotid atherosclerotic plaques, combined with other imaging to show that the particles are taken up almost exclusively by macrophages.
Michigan State postdoctoral fellow Mahsa Gifani is the first author of the paper, with Emory graduate student Devon Eddins as second author.
Looking ahead, Ghosn says he and Smith are considering modifying the nanoparticles so they can be detected by MRI (magnetic resonance imaging). They are also investigating carbon nanoparticles as a drug delivery vehicle into plaques or tumors. The particles could operate in a “Trojan Horse” sabotage mode, or modulate the activity of macrophages present in the plaques or tumors.
Read the original article on Emory Healthcare.
