Despite the progress made in current clinical cancer therapies, many cancer patients cannot benefit from them due to the swift development of drug resistance.
Nine out of 10 chemotherapy treatment failures occur during cancer invasion and metastasis associated with drug resistance, making drug resistance the bane of the cancer field. Presently, no therapeutic options exist to bypass this. Taiwanese company Acura NanoMedicine Inc. may just have the answer to this problem with their novel anti-cancer drug: AN-845, an innovative amalgamation of two repurposed drugs.
There are as many as 165 or more genes implicated in pancreatic tumour drug resistance. With one of the highest mortality rates among cancers, the five-year survival rate for treating patients is 5%.
By manipulating cancer cell metabolism and tumor microenvironment simultaneously, AN-845 acts as a "nuclear warhead," endorsed by Acura NanoMedicine Inc. as a super-effective anti-cancer drug. In experiments, the scientists of Acura NanoMedicine Inc. have observed firstly, AN-845 can completely block the growth of chemo-resistant pancreatic cancer in living animals. Secondly, when previously failed chemotherapeutic drugs combine with AN-845, AN-845 they can overcome drug resistance and restore the effectiveness of failed drugs. They can even go further by generating a synergistic anti-cancer effect to maximize therapeutic outcomes. The effect also extends beyond pancreatic cancer, right now Acura NanoMedicine are applying AN-845 to treat glioblastoma (an aggressive form of brain cancer), liver cancer, colon cancer among others.
AN-845 is a combination of two generic drugs that have proven safety. It can follow two fast-track regulations: 505(b)(2) and orphan drug. These two aspects made the development of this active pharmaceutical ingredient low-cost and fast, requiring under three years for clinical development.
Rethinking the approach to cancer treatment using Active-Targeting Nano-Vehicles
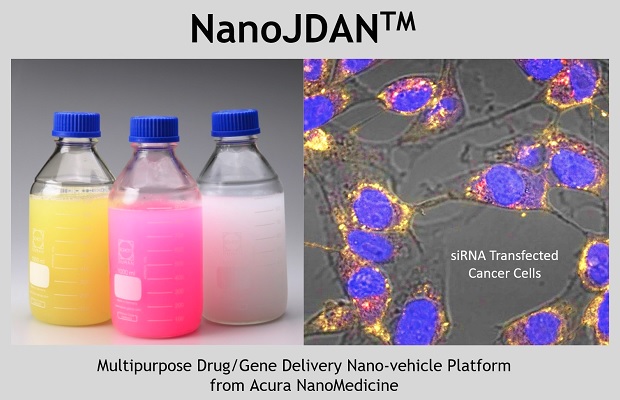
"Joint Direct Active-Targeting Nano-Vehicle" (NanoJDAN™) is employed as the "vehicle", comprising of cargos (small or large drug molecules), biomolecules (peptide, protein, aptamer, antibody) and bio-degradable polymers. NanoJDAN™ deliver drugs (and other molecules) to the target cells at an efficiency 80 to 100 times greater than traditional NPs. Where conventional antibody-drug conjugates can link less than ten drug molecules per antibody, NanoJDAN™ can encapsulate tens of thousands in one particle with high stability and tumor targeting ability. In cell therapy application, NanoJDAN™ can perform as an immuno-linker to attach and activate T/NK cells. When armed T/NK cells encounter cancer cells, the cancer-targeting moiety of NanoJDAN™ can grab the cells together to trigger the cellular-mediate tumour killing effect. Additionally, beyond Moderna/BNT's lipid nanoparticles, NanoJDAN™ can be used in DNA, mRNA and siRNA delivery for "Targeted" gene therapy.
"Acura NanoMedicine Inc. has expanded today's nanomedicine landscape and is committed to developing safe, effective and affordable medicines to treat cancers," says Brian Hsu, CEO, President, and Co-founder of Acura NanoMedicine. "Going beyond what is available, Acura NanoMedicine Inc.'s NanoJDAN™ can deliver mRNA to specific tissues without complex storage conditions. Making it an effective tool for performing next-generation gene therapy."
AN-845 has great potential to treat multiple cancers. NanoJDAN™'s platform can also be applied to multiple biomedical applications such as targeted cancer therapy, immuno-cellular therapy, and gene therapy. Moving forward, Acura NanoMedicine Inc. is looking at license-out and co-development opportunities with other companies.
Read the original article on PR Newswire.