We've all made the mistake of leaving a container of ice cream on the kitchen counter for a bit too long. Sure, you can refreeze the half-melted treat, but you may find that the texture is far more crunchy than delectably creamy afterward. The culprit is overly large ice crystals. Scientists at the University of Tennessee think they've found a plant-based additive to stop the formation of these crystals, and it's more effective and cheaper than the additives currently used by ice cream manufacturers. The researchers presented their work at this past week's meeting of the American Chemical Society in San Diego.
"Food science is not cooking," Tao Wu, a food scientist specializing in carbohydrate chemistry, said during a press conference. "It's a multi-disciplinary field that uses chemistry, biology, and engineering to solve real-world problems in the production of food. For instance, we must use good chemistry knowledge to produce high-quality ice cream."
The basic science involved in making ice cream is well known. (Physics students have even been known to use liquid nitrogen to make their own ice cream in the lab.) Just heat milk, cream, and sugar until the sugar dissolves; cool the mixture; and add any flavorings. Then slowly churn that mixture as it freezes. This adds air to the mixture, inflating the volume (overrun). The best ice creams, including gelato, have an overrun of less than 25 percent compared to cheap commercial ice creams, where the overrun can be as high as 100 percent. That higher overrun is why cheap ice creams melt more quickly and don't store as well. Finally, pack the soft ice cream mixture into containers for the final step in the process (hardening).
All ice cream contains ice crystals, but ideally, you want the smallest crystals possible to ensure a creamy rather than crunchy texture. The rapid chilling and churning process generally results in tiny seed crystals. Problems arise when ice cream melts and then refreezes—a process called recrystallization. If refrozen ice crystals become larger than 50 micrometers, the dessert will take on that undesirable crunchy texture.
To ensure ice cream stays creamy, manufacturers typically add emulsifiers like lecithin and stabilizers like guar gum, locust bean gum, carrageenan, and pectin. These stabilizers help the ice cream retain moisture during storage and slow the growth of ice crystals. However, "These stabilizers are not very effective,” Wu said. “Their performance is influenced by many factors, including storage temperature and time, and the composition and concentration of other ingredients. This means they sometimes work in one product but not in another.”
Further, according to Wu, it isn't clear exactly how these added ingredients interact and inhibit ice recrystallization. The focus of this latest research is to identify and test better alternatives.
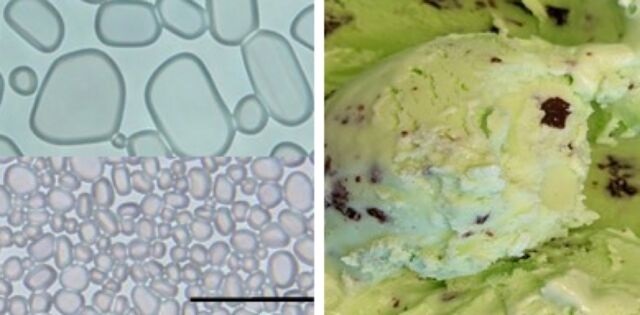
Adding cellulose nanocrystals prevents the growth of small ice crystals (bottom left) into the large ones (top left) that can make ice cream (right) unpleasantly crunchy.
Wu's graduate student, Min Li, said the team was inspired by the structure and functionality of antifreeze proteins found in certain species of fish, insects, and plants that thrive in sub-zero temperatures. These proteins have also been shown to prevent large ice crystals from forming. The proteins stick to the surface of ice crystals, keeping them from clustering into larger crystals. But there is a limited supply, and the proteins are very expensive, making them impractical for commercial use.
Prior research suggested that the anti-freezing capabilities of such proteins come from the fact that they have both a hydrophilic surface with an affinity for water and a hydrophobic surface that repels water. Because cellulose nanocrystals also have this so-called "amphiphilic" structure, Wu and his team thought they might also inhibit the growth of larger ice crystals. Unlike the antifreeze proteins and commercial stabilizers, "Nanocelluloses are abundant, renewable, and inexpensive," said Li.
The team conducted its experiments with a model ice cream. Initially, the added cellulose nanocrystals had no effect on the ice crystals compared to a control model ice cream with no added nanocrystals. That changed after the ice cream was stored for several hours; the nanocrystals stopped the growth of ice crystals entirely compared to the control model ice cream, in which larger ice crystals still formed. Further, the cellulose nanocrystals worked better than commercial stabilizers when the ice cream was exposed to fluctuating temperatures.
As for the underlying mechanism, the researchers found that the surface adsorption seemed to be the nanocrystals' secret to stopping ice recrystallization in its tracks—just like antifreeze proteins. "This completely contradicted the existing belief that stabilizers inhibit ice recrystallization by increasing viscosity, which was thought to slow diffusion of water molecules," said Li.
Wu estimates that nanocellulose-based antifreeze products could reach the market within the next three to five years, pending Food and Drug Administration approval. He does not expect there to be any issues with toxicity. "I believe they are safe to be added into food," he said.
The products could also prove useful in the cryopreservation of biological cells, tissues, and organs, which are susceptible to ice-crystal formation. "For example, in biotechnology and biomedicine, cells are typically stored in liquid nitrogen," said Wu. "During the storage, ice recrystallization can lead to cell damage or death. [Adding] ice recrystallization inhibitors during the cryopreservation process can increase the cell viability."
Read the original article on Ars Technica.
