In the fight against cancer, the development of efficacious drugs is only half the battle; equally important is how drugs may be delivered efficiently and safely to the diseased sites in the body. The challenge of drug delivery is especially pertinent for RNA therapeutics which target an important immuno-modulatory receptor, RIG-I. When activated by certain types of RNAs, the receptor can initiate immune responses to kill cancer cells. As RNAs are unstable and fragile by nature, RNA-based drugs must be packaged in suitable carriers to prevent degradation, and promote efficient uptake by target cancer tissues.
A study led by researchers at the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine)—in collaboration with the Lee Kong Chian School of Medicine, Nanyang Technology University (LKCMedicine, NTU) and A*STAR’s Genome Institute of Singapore (GIS)—demonstrated that nano-sized vesicles released by red blood cells are a viable platform for delivering immunotherapeutic RNA molecules to suppress breast cancer growth and metastasis.
Published in the Journal of Extracellular Vesicles, the study successfully delivered RIG-I-activating RNAs using small, lipid membrane-bound particles released by red blood cells, called red blood cell extracellular vesicles (RBCEVs), to suppress cancer progression. The team had also discovered in earlier studies that these vesicles are ideal therapeutic carriers with a natural ability to deliver bioactive molecules to many cell types.
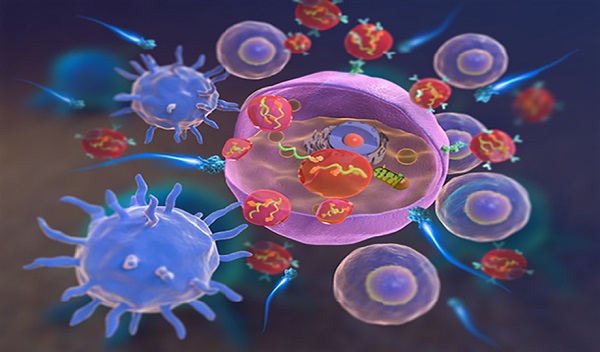
Extracellular vesicles deliver RIG-I agonists to fire up cancer cells and trigger immune attack.
Assistant Professor Minh Le from the Institute for Digital Medicine (WisDM) and Department of Pharmacology at NUS Medicine, who led the study, explained, “With the discovery of these vesicles’ ability to deliver therapeutics effectively to targeted receptors, we hope that our research can lead to better treatment outcomes for cancer patients. The correct homing of the therapeutics to diseased cells is also critical in minimising off-target effects that can result in toxicity.”
To further examine the function of RBCEVs in carrying a broader range of therapeutics to more cancer cell types, the team plans to conduct further research in collaboration with the National University Cancer Institute and Cancer Science Institute of Singapore. Concurrently, RBCEV technologies are under intensive research at Carmine Therapeutics, an EVX Ventures company which aims to develop the next generation of gene therapy based on RBCEVs for treatments of rare diseases and cancer.
Professor Dean Ho, Director of WisDM, added, “Our team at WisDM is committed to bridging medical innovation with clinically-relevant impact. The findings by Asst Prof Le and collaborators represent major advances in RNA-based therapy and nanomedicine that can be realised through multidisciplinary ecosystems.”
WisDM is one of ten Translational Research Programmes at NUS Medicine aimed at creating a strong and coherent scientific base to deliver impactful and meaningful research outcomes for the School and Singapore’s health system.
Read the original article on National University of Singapore.