A research team led by Professor Seo Dae-ha of the Department of Physics and Chemistry at DGIST (President Kuk Yang) developed an optical microscopy that can control and observe electron transfer and transfer in complex chemical reactions occurring in nano-catalysts. This technology is expected to provide an experiment strategy based on system chemistry, a new experiment strategy for precisely studying photocatalysts at the single particle level.
Plasmonic metals at the nanometer level, such as gold, exhibit high light absorption rate in a wide place within the range of visible light. They are combined with semiconductor photocatalysts to act as a medium to increase light absorption. Excitation occurs in which electrons gain energy and move as a reaction to light absorption, and it appears through various paths depending on the size of the metal and the wavelength of the light. There are various hypotheses on the effect of this electron movement as a catalyst. The research team was able to test the hypotheses and reveal how electrons transfer by developing a new microscope that is experimentally simpler and more sophisticated than the conventional method of observing chemical reactions.
Professor Seo Dae-ha's research team developed hybrid nanoparticles (for example, 'gold/copper oxides', a combination of gold and copper oxides), and lasers of different wavelengths (colors) (i.e., lasers A, B, and C are A+B, A+C ... A+B+C) were combined into a new form, respectively, to investigate the reaction between them to test various hypotheses on the electron excitation phenomenon through experiments and verify them one by one. Through this process, the team was able to selectively induce electron excitation in gold nanoparticles, and quantitatively analyze their contributions by evaluating the increase in the reactivity of the catalyst. In addition, the team confirmed that these excited electrons were transferred to the semiconductor to increase stability and reactivity at the same time.
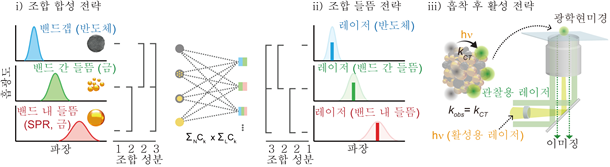
Schematic diagram of the strategy for evaluating catalytic activity at the single particle level.
“The observational technology reported here is a technology that observes chemical reactions with high precision, efficiency, and low cost,” said Professor Seo Dae-ha of the Department of Physics and Chemistry at DGIST, while adding, “It is expected that it will contribute to the sophisticated design of catalysts and will be applied as a sophisticated evaluation and control technology using nanoparticles for pharmaceuticals.”
Meanwhile, this research was carried out with support of the National Research Foundation's Leading Researcher Support Project, Leading Research Center, Biomedical Technology Development Project, and DGIST's Grand Challenge Research Innovation Project. The research results were published in the top international academic journal in the field of chemistry, Chem (IF=22.8), online on June 27, 2022.
Read the original article on Daegu Gyeongbuk Institute of Science & Technology (DGIST).