2023-01-16
Visited : 2797
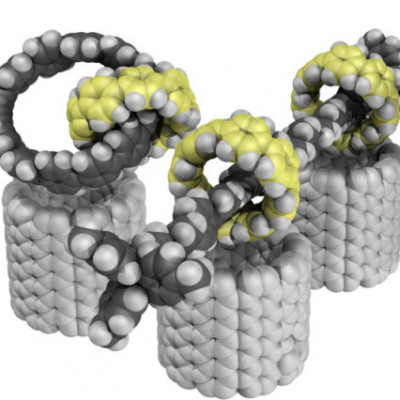
There’s a new nanomaterial on the block. UO chemists have found a way to make carbon-based molecules with a unique structural feature: interlocking rings.
Like other nanomaterials, these linked-together molecules have interesting properties that can be “tuned” by changing their size and chemical makeup. That makes them potentially useful for an array of applications, such as specialized sensors and new kinds of electronics.
“It's a new topology for carbon nanomaterials, and we’re finding new properties that we haven't been able to see before,” said James May, a graduate student in chemistry professor Ramesh Jasti’s lab and the first author on the paper. May and his colleagues report their findings in a paper published Jan. 12 in Nature Chemistry.
Though other labs have also synthesized various types of interlocking molecules, the Jasti lab’s method allows carbon nanotube-like structures to be linked together. It will allow chemists to make many different variations on the structure and more fully explore the properties of the new materials.
“You can create structures you can't with other methods,” Jasti said.
For example, his team used the approach to make three interlocked rings, as well as a rod-like structure with multiple rings that can slide up and down. The advance grew out of Jasti’s work on nanohoops, rings of carbon atoms that are a pared-back variation of long, skinny carbon nanotubes.
“Because we're able to make these circular structures at will, I started thinking, could you make things that just don't exist in nature?” Jasti said. “That's where this idea of interlocking rings came in.”
Finding a series of chemical reactions that could generate the complicated ring structures took a creative approach. Their solution hinges on adding a strategically placed metal atom to one ring. That metal kickstarts the chemical reaction to make the second ring, forcing it to happen inside the first ring. Once that reaction happens, the second ring is trapped, locked together with the first ring.
“We’re able to get chemistry to happen inside of a space where it might never occur,” May said.
The interlocked molecules behave differently if their size changes or if the rings are arranged differently or if different chemical elements are thrown into the mix. By making nanoscale adjustments, scientists could enhance the material to do exactly what they want it to do. Because the class of materials is so new, scientists are still figuring out all of the possibilities.
But Jasti’s team is particularly interested in their potential as sensors, where a change in the position of the rings in response to a particular chemical could lead to a fluorescent glow.
They also could be used to create flexible electronics or dynamic biomedical materials.
“Typical carbon nanomaterials like carbon nanotubes, graphene or even diamond are static materials,” he said. “Here, we have created new types of carbon nanomaterials that maintain their fascinating electrical and optical properties but now have capability to do things like rotate, compress, or stretch.”
Read the original article on University of Oregon.