Researchers from the Disruptive & Sustainable Technologies for Agricultural Precision (DiSTAP) Interdisciplinary Research Group (IRG) of Singapore-MIT Alliance for Research and Technology (SMART), MIT’s research enterprise in Singapore and their collaborators from Temasek Life Sciences Laboratory (TLL) have developed the first ever nanosensor that can detect and distinguish gibberellins (GAs), a class of hormones in plants that are important for growth. The novel nanosensors are non-destructive, unlike conventional collection methods, and have been successfully tested in living plants. Applied in the field for early-stage plant stress monitoring, the sensors could prove transformative for agriculture and plant biotechnology, giving farmers interested in high-tech precision agriculture and crop management a valuable tool to optimise yield.
The researchers designed near-infrared (NIR) fluorescent carbon nanotube sensors that are capable of detecting and distinguishing two plant hormones, GA3 and GA4. Belonging to a class of plant hormones known as gibberellins (GAs), GA3 and GA4 are diterpenoid phytohormones produced by plants that play an important role in modulating diverse processes involved in plant growth and development. GAs are thought to have played a role in the driving forces behind the ‘green revolution’ of the 1960s, which was in turn credited with averting famine and saving the lives of many worldwide. The continued study of gibberellins could lead to further breakthroughs in agricultural science and have implications for food security.
Climate change, global warming and rising sea levels cause farming soil to get contaminated by saltwater, raising soil salinity. In turn, high soil salinity is known to negatively regulate GA biosynthesis and promote GA metabolism, resulting in the reduction of GA content in plants.
The new nanosensors developed by the SMART researchers allow for the study of GA dynamics in living plants under salinity stress at a very early stage, potentially enabling farmers to make early interventions when eventually applied in the field. This forms the basis of early stage stress detection.
Currently, methods to detect GA3 and GA4 typically require mass spectroscopy-based analysis, a time-consuming and destructive process. In contrast, the new sensors developed by the researchers are highly selective for the respective GAs and offer real-time, in vivo monitoring of changes in GA levels across a broad range of plant species.
Described in a paper titled “Near-Infrared Fluorescent Carbon Nanotube Sensors for the Plant Hormone Family Gibberellins” published in the journal Nano Letters, the research represents a breakthrough for early-stage plant stress detection and holds tremendous potential to advance plant biotechnology and agriculture. This paper builds on previous research by the team at SMART DiSTAP on single-walled carbon nanotube-based (SWNT-based) nanosensors using the corona phase molecular recognition (CoPhMoRe) platform.
Based on the CoPhMoRe concept pioneered by the Strano Lab at MIT, the novel sensors are able to detect GA kinetics in the roots of a variety of model and non-model plant species, including Arabidopsis, lettuce and basil, as well as GA accumulation during lateral root emergence, highlighting the importance of GA in root system architecture. This was made possible by the researchers’ related development of a new coupled Raman/NIR fluorimeter that enables self-referencing of nanosensor NIR fluorescence with its Raman G-band, a new hardware innovation that removes the need for a separate reference nanosensor and greatly simplifies the instrumentation requirements by using a single optical channel to measure hormone concentration.
Using the reversible GA nanosensors, the researchers detected increased endogenous GA levels in mutant plants producing greater amounts of GA20ox1, a key enzyme in GA biosynthesis, as well as decreased GA levels in plants under salinity stress. When exposed to salinity stress, researchers also found that lettuce growth was severely stunted - an indication that only became apparent after 10 days. In contrast, the GA nanosensors reported decreased GA levels after just 6 hours, demonstrating their efficacy as a much earlier indicator of salinity stress.
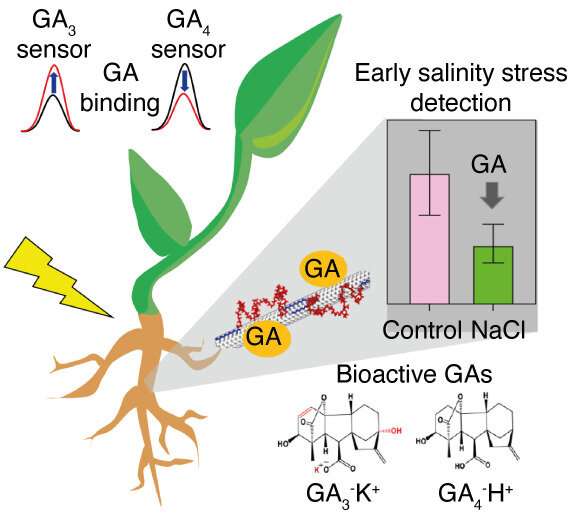
Illustration of GA detection in living plants using near-infrared fluorescent carbon nanotube sensors for early indication of salinity stress
“Our CoPhMoRe technique allows us to create nanoparticles that act like natural antibodies in that they can recognize and lock onto specific molecules. But they tend to be far more stable than alternatives. We have used this method to successfully create nanosensors for plant signals such as hydrogen peroxide and heavy-metal pollutants like arsenic in plants and soil. The method works to create sensors for organic molecules like synthetic auxin - an important plant hormone - as we have shown. This latest breakthrough now extends this success to a plant hormone family called gibberellins - an exceedingly difficult one to recognize,” said co-corresponding author, DiSTAP co-lead Principal Investigator Professor Michael Strano and Carbon P. Dubbs Professor of Chemical Engineering at MIT. “The resulting technology offers a rapid, real-time, and in vivo method to monitor changes in GA levels in virtually any plant, and can replace current sensing methods which are laborious, destructive, species-specific and much less efficient.”
Dr Mervin Chun-Yi Ang, Associate Scientific Director at DiSTAP and co-first author of the paper, added, “More than simply a breakthrough in plant stress detection, we have also demonstrated a hardware innovation in the form of a new coupled Raman/NIR fluorimeter that enabled self-referencing of SWNT sensor fluorescence with its Raman G-band, representing a major advance in the translation of our nanosensing toolsets to the field. In the near future, our sensors can be combined with low-cost electronics, portable optodes, or microneedle interfaces for industrial use, transforming how the industry screens for and mitigates plant stress in food crops and potentially improving growth and yield.”
The new sensors could yet have a variety of industrial applications and use cases. As TLL Principal Investigator, NUS Adjunct Assistant Professor Daisuke Urano and co-corresponding author of the paper explained, “GAs are known to regulate a wide range of plant development processes, from shoot, root, and flower development, to seed germination and plant stress responses. With the commercialisation of GAs, these plant hormones are also sold to growers and farmers as plant growth regulators to promote plant growth and seed germination. Our novel GA nanosensors could be applied in the field for early-stage plant stress monitoring, and also be used by growers and farmers to track the uptake or metabolism of GA in their crops.”
The design and development of the nanosensors, creation and validation of the coupled Raman/NIR fluorimeter and related image/data processing algorithms, as well as statistical analysis of readouts from plant sensors for this study was done by SMART and MIT; while TLL was responsible for the design, execution and analysis of plant-related studies, including validation of nanosensors in living plants. The research is carried out by SMART and supported by NRF under its Campus for Research Excellence And Technological Enterprise (CREATE) programme.
Read the original article on SMART.
