MXenes are a large family of two-dimensional (2D) inorganic compounds derived from transition metal carbides, nitrides, or carbonitrides, which have been first described in 2011. These compounds were proposed to be called “MXene” to emphasize its graphene-like morphology.
The first 2D MXene (Ti3C2) was isolated at Drexel University in 2011. This material, which is a layered bulk material analogous to graphite, was derived from its 3D phase by the room temperature exfoliation of Ti3AlC2 MAX in hydrofluoric acid. MAX is a large family of ternary carbide with the general formula Mn+1AXn. M represents an early transition metal element, such as Ti, A stands for an element such as aluminum or silicon, and X refers to C, N, or their blends. MXene multilayer flakes are usually produced by the selective removal of the A layers in MAX phases. The removal of the Al layers dramatically weakens the interactions between the Mn+1Xn layers that, in turn, allows them to be readily separated.
It is difficult to determine the exact thickness of an MXene layer, however, in all cases, the individual MXene layer thickness is around 1 nm, while their lateral dimensions can reach tens of microns (Figure 1).

Figure 1- Scanning electron microscopy (SEM) image of exfoliated MXene nanosheets.
(ACS Nano 2012, 6, 1322).
Since the discovery, material scientists have either determined or predicted the stable phases of more than 200 different MXenes based on the combinations of various transition metals such as Ti, Mo, V, and Cr, as well as their alloys with C and N.
The electronic properties of MXenes are of particular interest as they can, in principle, be tuned by changing the MXene elemental composition and/or their surface terminations. This new family of 2D structures with a wide range of chemistries can improve our understanding of differences between properties of 2D and 3D materials and result in new applications. Their distinctive properties include high electrical conductivity and the hydrophilic nature of their hydroxyl or oxygen terminated surfaces, a unique combination which earned them a nickname “conductive clays.”
The mechanical properties of MXenes are also of great interest as the M–C and/or M–N bonds are some of the strongest known. Moreover, they are chemically stable. MXenes can enhance electrical transport in the composite materials. They have adjustable electronic properties for metallic or semiconducting conductivity, tunable work functions, high optical transmittance, and outstanding heat conversion efficiency. Given these properties, MXenes show exceptional promise in many applications.
Extensive experimental and theoretical studies have shown their exciting potential for energy conversion and electrochemical storage. The former includes electrodes in rechargeable lithium- and sodium-ion batteries, lithium-sulfur batteries, and supercapacitors. The latter contains photocatalytic fuel production, hydrogen evolution from water splitting, and carbon dioxide reduction. They also showed potential for the photocatalytic degradation of organic pollutants in water, such as dye waste along with their promise as catalysts for ammonium synthesis from nitrogen.
These compounds have recently attracted scientific consideration so that 894 related articles have been published since 2018 compared to 1399 articles (102 review articles) since their discovery. Publications in such a field are growing, and the number of articles published in the first half of 2019 is comparable to the publications in 2018 (figure 2). Journal of Materials Chemistry A, ACS Nano, and Nanoscale have the largest share in terms of MXenes related publications.
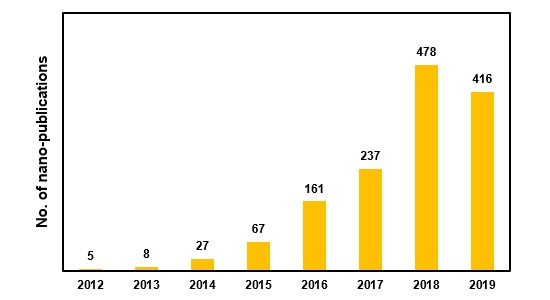
Figure 2- Number of nano-publications by year since the discovery of MXenes nanostructures.
Many aspects of MXenes are still unknown. In the future, theoretical predictions will continue to lead to the expansion of this family through different elemental combinations, resulting in new properties and finally in a novel, high-tech applications.


