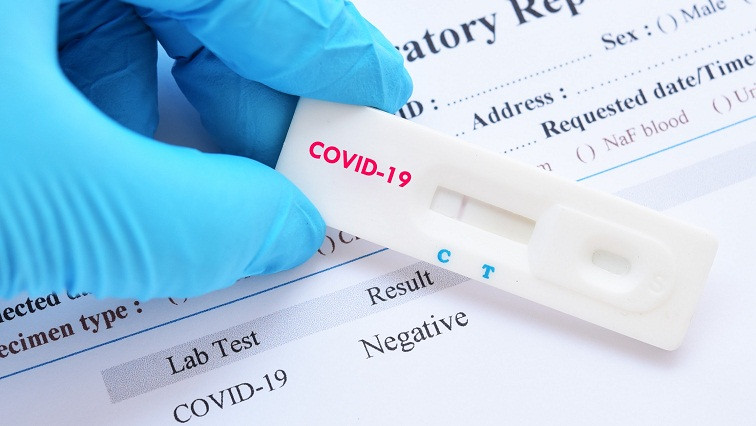
Using easy to collect samples, the tests detect the IgM and IgG antibodies response to the coronavirus, to identify if patients have contracted COVID-19 within minutes of testing.
The technology, which has already been used in China, will improve the detection rate of patients carrying COVID-19, allowing doctors to test suspected carriers as soon as 2 days after suspected exposure.
The antibody test, which was approved for sale in European markets last week, promises to ease pressure on labs by providing rapid point-of-care diagnostics. This will assist healthcare professionals to clinically assess patients with and without symptoms, so they can provide vital care to those most in need even quicker.
Total coronavirus cases are set to surpass 240,000 worldwide today, and having been granted CE mark certification, the tests can be made available immediately, with manufacturing being increased to meet demand over the coming weeks.
Today, British Prime Minister, Boris Johnson, confirmed interest in procuring COVID-19 tests which are as “simple as a pregnancy test, which can tell whether you have had the disease and in its early days, but if it works as its proponents claim, then we will buy literally hundreds of thousands of these kits as soon as practicable.”
He continued, “Because obviously it has the potential to be a total gamechanger.”
Global not-for-profit, The World Nano Foundation (WNF), through its partners, has formed a partnership with the Chinese developer of the test, to make them available across Europe.
WNF believe that the tests should be offered to Governments first, as they are best positioned to prioritise the application of the tests, rather than have the supply hoovered up indiscriminately by the market.
For more information about the 15-minute COVID-19 tests, please contact Paul Sheedy, Co-Founder of The World Nano Foundation: paul.sheedy@worldnanofoundation.com
Read the original article on World Nano Foundation.