Entos Pharmaceuticals (Entos), a healthcarebiotechnology company developing next generation nucleic acid medicines with their Fusogenix delivery platform announced a partnership with PrecisionNanoSystems, Inc., (PNI), a global leader in enabling transformative nanomedicines, to produce clinica lgrade vaccines and therapeutics using PNI’s NanoAssemblr™ GMP System.
Entos develops next generation nucleic acid-based therapies using the proprietary Fusogenix drug delivery system, a proteo-lipid vehicle (PLV) that delivers molecules,intact and unmodified, directly into the cytosol of target cells. Entos has recently launched a DNA vaccine rapid prototyping project around the development of a pan-coronavirus DNA vaccine, with the aim of broad availability within one year.
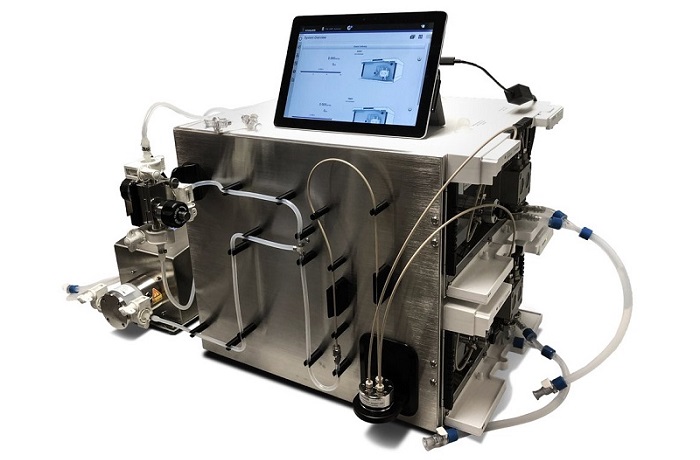
To reach this ambitious target, Entos will incorporate PNI’s NanoAssemblr GMP System into the manufacturing suite at the Alberta Cell Therapy Manufacturing (ACTM) Facility, located in the University of Alberta’s Li Ka Shing Centre for Health Research Innovation. This will enable the production of clinical grade Fusogenix genetic nanomedicines such as Covigenix, Entos’s pan-coronavirus Fusogenix DNA vaccine.
James Taylor, Co-Founder and CEO of PNI said, “We’re excited to rapidly deploy our NanoAssemblr GMP System and NxGen microfluidic technology at ACTM for Entos to scale up and manufacture their DNA vaccine against COVID-19 using their drug delivery system.”
“PNI is globally renowned for their NanoAssembr suite of products that enable high quality, consistent nanoparticle formation and seamless scale up from the laboratory to GMP manufacturing for the clinic,”said Dr. John Lewis, CEO of Entos, “using their GMP System will let us meet the challenge of producing our pan-coronavirus DNA vaccine at large scale and in as short a time as possible, while exceeding all safety and quality requirements.”
PNI’s proprietary NanoAssemblr GMP System is a modular solution to enable rapid deployment and configuration to meet the requirements at each scale of clinical manufacturing. PNI’s Clinical Solutions team will perform technology transfer of the GMP System to ACTM and support accelerating Entos’ COVID-19 vaccines and therapeutics, significantly reducing the time needed to deliver these needed programs to the public.
Read the original article on Entos Pharmaceuticals.