Another intriguing aspect of nanostructuring active Li-ion materials is their capability to continuously store more lithium (thus energy) per unit formula than their bulk, micron-sized counterparts.
Such phenomenon has been already demonstrated in thin films or nanoparticles of MnO2, LiMn2O4 and Li4Ti5O12, upon lowering their thickness below some tens of nanometers.
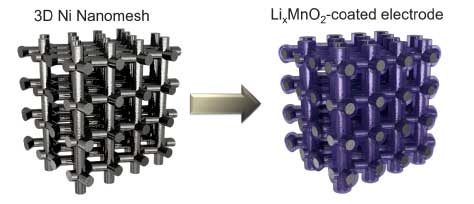
Fig. 1. Schematic showing Li-ion electrode based on 3D Ni nanomesh current collector coated with LixMnO2 active material.
The increased rechargeable capacity can stem from better accommodation of mechanical stress during cycling, change in surface crystal composition or additional lithium storage at the extended surfaces of nanostructured materials.
Gaining access to the additional active capacity is particularly important for reaching high volumetric energy in nanostructured electrodes, which typically suffer from low packing density of energy-storing components.
In our work (Journal of Materials Chemistry A, "Interconnected Ni nanowires integrated with LixMnO2 as fast charging and high volumetric capacity cathodes for Li-ion batteries"), we utilized 3D-interconnected Ni nanowire meshes (or simply, nanomeshes) as current collectors and structural scaffolds for building nanostructured Li-ion electrodes. This material, described in our previous publications, exhibits a semi-ordered structure and is characterized by some of the highest combined porosity and surface-to-volume ratio among macroporous metals.
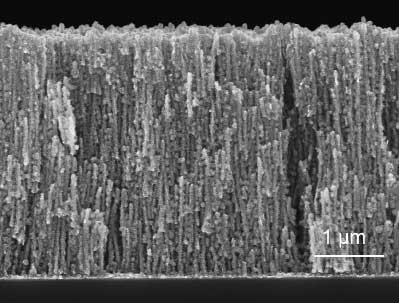
Fig. 2. Scanning electron microscopy cross-section image of the LixMnO2-coated nanomesh electrode.
The high porosity of the current collector allows packing more active material into its structure while the high surface area allows distributing the active material over a very thin, few nanometers layer.
As a consequence of improved loading of the active material and higher utilization of its active capacity, the Li-ion electrodes made of nanomesh coated with LixMnO2 active material exhibit rechargeable volumetric capacity of about 200 mAh/cm3, which is among the highest among monolithic 3D Li-ion cathodes described so far.
Also, because the 3D nanomesh provides uniform access of electrons to the entire volume of the active material and ionic transport in the nanometer-thick active layer is unimpeded, the cathodes can be cycled at significantly high rates, charging to 50% of nominal capacity in 75 seconds.
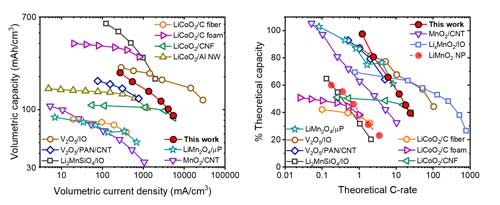
Fig. 3. Performance of the nanomesh cathodes vs. other 3D-monolithic Li-ion cathodes in terms of volumetric capacity at different volumetric current densities (left) and utilization of active material at different C-rates (right).
From the technological point of view, fabrication of the electrodes is relatively fast, uses cheap chemicals and processes and can allow making nanomesh electrodes supported on a few micron-thick flexible nickel foils.
This offers potentially tremendous saving in the total electrode volume compared to the typical monolithic 3D electrodes that, in most cases, are supported on hundreds-of-microns thick rigid supports.
Although the small overall thickness of the nanomesh used in the current work makes them not yet applicable in practical batteries, we hope that our work can inspire future development of cheap, optimally-structured 3D Li-ion electrodes with high energy and power density.
Read the original article on Nanowerk.