Investigating nanomaterials represents an extreme challenge of scale. Vanishingly small, nanomaterials are made of particles less than 100 nanometers across. A nanometer is one-billionth of a meter. A sheet of paper, by comparison, seems grossly thick: 100,000 nanometers wide.
They are ancient and natural, part of Earth’s landscape for at least 4.5 billion years. Sand, for instance, is a nanomaterial created by erosion. However, nanomaterials are also creatures of the Industrial Age. For the last 250 years, they have been unintentionally shed into the air, water, and land as a result of mining, farming, manufacturing, transportation, and other human activities.
In the past 50 years or so, nanomaterials have been intentionally engineered to leverage their distinct chemical, physical, and electrical properties. Ranging from medicine and cosmetics to energy and aerospace, nanomaterials can be found in paint, prescription drugs, toothpaste, batteries, bandages, sunscreen, and even socks.
Engineered nanoparticles reach the environment at a rate of about 0.3 teragrams per year. A teragram is a measure of weight equal to 1 trillion grams. That’s a tiny fraction compared to natural nanoparticles, such as the dust created by eons of weathered rock. But every year, more nanoparticles are manufactured for more uses.
In medicine, for instance, nanomaterials can shuttle drug molecules into the body with an impressively large surface-to-mass ratio. Their small size enables them to penetrate target cells and organs with precision.
Nanoparticle technologies hold the promise to improve processes that already exist―for instance, industrial catalysis will become faster, more efficient, and more sustainable. In catalysis, nanomaterials mean less material is required―and more of that material is already in a reactive state, ready to speed chemical transformations.
Researching nanoparticles
In the summer of 2020, PNNL was named a partner institution in a continuing five-year, $20 million NSF grant for molecular-level research on the transformation, interactions, and impact of various nanomaterials released into the environment. Twelve institutions in nine states are taking part. Among them, PNNL is the only U.S. Department of Energy national laboratory.
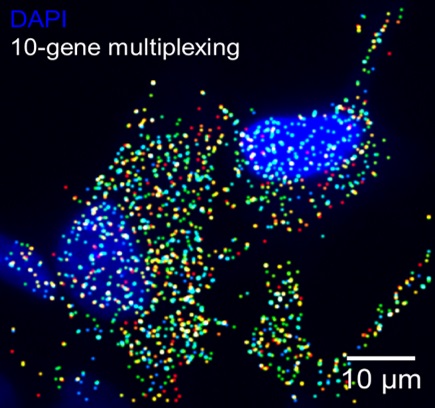
Expression level of 10 genes was quantified simultaneously in response to cellular exposure to lithium cobalt oxide nanoparticles. These nanoparticles are being used in lithium batteries and are likely to reach the environment after disposal. The cell nuclei are shown in blue, and the different color dots are transcripts for the different genes.
This new grant is from the NSF Division of Chemistry, which continues to fund the Center for Sustainable Nanotechnology (CSN), a multi-institutional research center based at the University of Wisconsin-Madison. The CSN was founded in 2012, with PNNL as an inaugural partner.
Researchers at PNNL will use staff expertise and instrumentation at EMSL—the Environmental Molecular Sciences Laboratory, a U.S. Department of Energy user facility located on the PNNL campus. EMSL specializes in biological and biogeochemical investigations at molecular scales.
Health concerns
For about the last 20 years, the modern ubiquity of nanomaterials has made them the subject of intense study regarding potential health effects. One concern is that particles so small can enter the body and slip easily into the bloodstream or lung tissue.
The CSN is focused on the molecular-level transformation and effects of these technologically relevant nanomaterials. Specifically, researchers funded by the renewed grant aim for a predictive understanding of the fate of engineered nanomaterials once they enter the air, water, soil, and the human body.
“Engineered nanomaterials have not been in the environment for a long time,” said University of Wisconsin-Madison professor of chemistry and CSN director, Robert Hamers. “So biological systems have not adapted to challenges they present, leaving the potential for greater environmental impacts.”
Researchers at PNNL and elsewhere ask: What molecular events occur at the interface of nanomaterials and biological systems in the environment? How can negative impacts be mitigated?
PNNL plays a role
At PNNL, the NSF grant’s co-principal investigator is scientist Galya Orr, a molecular biologist active in the CSN research for almost 8 years.
“A molecular level understanding of these processes and interactions will enable the design and synthesis of safer, next-generation nanomaterials,” she said―nanomaterials that maintain “high performance while minimally affecting biological systems.”
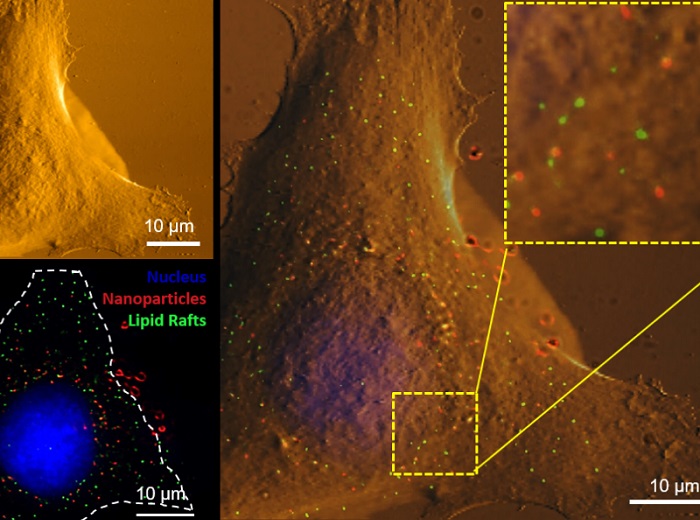
Using correlative atomic force microscopy (upper left) and super resolution fluorescence microscopy (lower left) to image the same cell, certain nanoparticles (red) were found to prefer binding to specialized nanodomains, called lipid-rafts (green), at the cell membrane. The overlaid image is shown on the right, where the inset in the upper right corner shows a zoom-in of the area marked by the dashed square. The cell nucleus is shown in blue.
One core CSN mission, Orr said, is “to identify critical molecular and biochemical processes that occur at the interface between nanomaterials and biological systems.”
That means using representative cell types and organisms to focus on molecular responses and impacts in the environment. In that sphere, PNNL benefits from using EMSL and its unique staff and instrumentation. Orr pointed to EMSL’s expertise in single molecule-based sub-diffraction fluorescence imaging in living cells. These biophysical microscopy techniques can detect and analyze single fluoresced molecules in a way that barely perturbs biological specimens.
This capability, said Orr, enhances the study of molecular and biochemical processes inside intact hydrated cells. Importantly, such studies take place at nanometer spatial resolutions and with high chemical selectivity―both EMSL specialties. In analyzing single cells quantitatively, scientists at PNNL also use a related EMSL capability, said Orr: “super resolution and other fluorescence microscopy techniques” in combination with atomic-force microscopy.
Atomic-force microscopy is used to scan the surface of a cell to study its topography. The technology deploys a sharp-tipped cantilever that scans as it is deflected toward a sample by atomic forces. The resulting image has a resolution measured in fractions of a nanometer. With such technologies at hand, the PNNL-EMSL technology nexus enhances the study of molecular and biochemical processes at the interface of intact cells and nanomaterials.
The promise of nanotechnology
CSN, the Center for Sustainable Nanotechnology research, is one of NSF’s Centers for Chemical Innovation, which are tasked to perform fundamental research on ”grand challenges in the field,” said David Berkowitz, director of the Division of Chemistry, which oversees such centers. He called the CSN’s research “a key element in the industries of the future.” With good reason.
At sizes less than 100 nanometers, and with versatile shapes and material properties, nanoparticles have been incorporated into composite materials, such as the silica nanoparticles used in modern rubber tires. Nanoclays improve the strength of polymers and make a future of non-petroleum polymers possible.
Nanomaterials also hold promise as neutralizers of organochlorine compounds in contaminated aquifers and underlie technologies for quantum imaging dots, molecular magnets, and the nanowires needed for molecular electronics. Moreover, nanomaterials have huge surface areas relative to their sizes, a property desirable in catalytic applications, for instance. A single teaspoon of silica nanospheres, each 10 nanometers in diameter, has more surface area than a dozen tennis courts used to play doubles.
To add to their versatility, nanoparticles can be organic, such as liposomes and branched molecules called dendrimers. They can also be inorganic, such as quantum dots and the gold nanoparticles used in sensors, probes, and diagnostic devices.
For all their promise, and for their increasing utility in industry and medicine, nanoparticles evoke caution. Not only can they slip easily into lung tissue or the bloodstream, they can interact with living tissue at the level of a single cell.
Chameleon particles
It is in that realm of nanoparticle interactions with single living cells that Orr and her team excel. In the past, Orr has characterized nanoparticles―with their huge surface-to-mass ratios and reactive natures―as “tiny chameleons” that can change rapidly once they interact with biological systems. That rapid reactivity makes their behavior difficult to predict once they have been inhaled or ingested, as they have the ability to impersonate cellular components or oxygen-delivery systems such as vesicles in the lungs.
Their unpredictability is one of the cruxes of what Berkowitz called “the grand challenge” of understanding the interchange of biological systems with nanoparticles. It turns out that once inside organisms or cells, nanoparticles are chemical pinballs. Will they racket around causing damage―or win the game? Will they be biocompatible or bio-toxic?
For living cells, the answer is: either, or both―a slippery reality because of the ever-changing reactivity and chemical states of nanoparticles. In a blog for CSN, Orr discusses two examples: cerium dioxide nanoparticles, an oxide of the rare-earth metal cerium used as a polishing agent, and zinc oxide nanoparticles used in sunscreen.
Measuring the evasive
The challenge then becomes how to measure the changing chemical states of those nanoparticles within fragile, hydrated, living cells. To this end, Orr and her research team use techniques such as super-resolution fluorescence microscopy to study nanoparticles within an organelle, with nanometer resolution.
But it’s difficult.
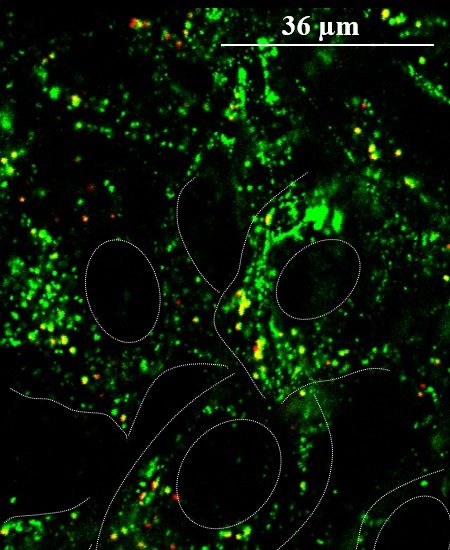
Using fluorescence microscopy to image cells exposed to positively charged silica nanoparticles, researchers found the nanoparticles (red) tend to aggregate in small intracellular vesicles together with syndecan-1 (green), a cell membrane proteoglycan that interacts with the nanoparticles and mediates their movement into the vesicle. Cells and nuclei are outlined with dashed white lines.
For one, the chemical properties of nanomaterials seem to depend on where the particles are within a single cell. These properties also depend on how quickly the nanomaterials degrade. When that happens, Orr’s team has discovered, nanoparticles generally cause the most harm.
Orr and her team also use x-ray microscopy to acquire what is called morphometric data to visualize what happens within and outside living cells. The technique pairs a powerful microscope with synchrotron-generated x-rays at Lawrence Berkeley National Laboratory’s Advanced Light Source.
With another visualization technique, PNNL researchers use a differential interference contrast microscope to capture the movement of nanoparticles as they enter cells. In another CSN blog post, which includes a molecule-scale movie, Orr compares the movement to nanoparticles surfing waves.
In all these ways, PNNL contributes to unlocking the puzzle of nano-bio interactions, a novel dynamic that is both a boon and a worry. In the end, engineered nanoparticles represent a growing issue in need of attention―the kind that the NSF Center, PNNL, Orr, and others can give it.
Read the original article on Pacific Northwest National Laboratory (PNNL).







