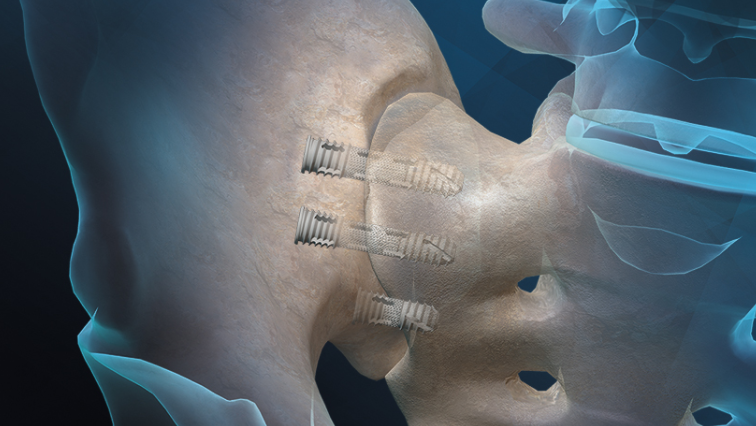
“The FIREBIRD SI Fusion System with NANOVATE™ technology is one of the many new differentiated solutions that Orthofix is proud to highlight during the upcoming North American Spine Society (NASS) 2020 virtual annual meeting,” said Kevin Kenny, Global President of Orthofix Spine. “The clearance of the nanotechnology feature gives us the opportunity to educate surgeons about the unique benefits of the system’s nano-surface. Created through a proprietary manufacturing process, the FIREBIRD SI Fusion System is the result of our intense focus on bringing innovations to the market to enable surgeons to meet the needs of their patients.”
The FIREBIRD SI Fusion System is implanted through a minimally invasive procedure that involves inserting two to four bone screws across the SI joint to stabilize the joint during the fusion process. The system’s 3D-printed mid-shaft porous region is designed to allow for bone through growth through the device to aid in the fusion process for patients being treated for pain and dysfunction of the SI joint.”
Featuring a cannulated screw design, the system enables surgeons to pack the device with autograft and/or allografts to help promote bone fusion. The FIREBIRD SI screws are available in an assortment of lengths and diameters to address a variety of patient anatomies. Orthofix offers allograft solutions such as the Trinity ELITE™ allograft with viable cells through a partnership with MTF Biologics.
Common causes that may lead to SI joint dysfunction and pain include trauma, lifting or twisting, pregnancy, natural childbirth, degeneration from previous lumbar spine surgery, stresses to the joint due to leg length differences, joint replacement or scoliosis among others. Published clinical literature indicates that sacroiliac joint pain is estimated to affect between 15 and 30 percent of individuals with chronic low back pain.
Read the original article on Business Wire.