While most COVID-19 cases are mild or asymptomatic, in some cases, the disease causes serious or critical complications, including multi-organ dysfunction and death. The only known way to deal with this virus at present is via non-pharmaceutical interventions (NPIs) such as social distancing, case isolation and contact tracing – and even national or regional lockdowns. However, these are associated with heavy economic disruptions, making the development of effective pharmacological approaches to the pandemic vital.
Human antibody
A human antibody or immunoglobulin molecule is a Y-shaped structure. It comprises four polypeptides, two heavy and two light chains. This allows antibodies to bind to antigens, on the one hand, while mediating their biological activity on the other. Both functions are traceable to different regions of the antibody, the fragment antigen-binding (Fab) and fragment crystallizable (Fc) regions.
The Fc region binds to the cell surface receptors called Fc receptors, and to some complement components. This links antibody binding to immune activation.
Nanobodies
The current study deals with the quest to develop nanobodies (Nbs) or VHHs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which are a novel class of proteins based on antibody fragments with only one domain. They are derived from the heavy chain variable domains of camelids such as camels, llamas and alpacas, lacking a light chain component.
The Nb domains have full antigen-binding potential, with high affinity for their antigens. These have the smallest intact antigen-binding domain (about 15 kDa) and are only about 110 residues in length.
Nb domains have immense potential as therapeutic proteins for several reasons: their small size, stability, high solubility, cost-effective production in a yeast vector and high tissue penetration due to their low molecular weight. However, at present, they cannot be administered systemically because of low gut absorption.
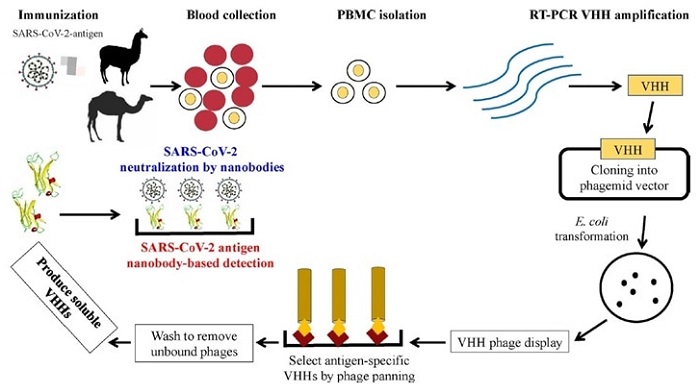
Schematic representation of the heavy-chain variable domains (VHHs) generation process and their potential applications as therapeutic agents and as diagnostic tools for COVID-19 pathology. Blood is collected from SARS-CoV-2 antigen-immunized camelids to isolate PBMCs. RNA is extracted from PBMCs followed by RT-PCR to amplify VHH. The VHH DNA sequence is ligated into a phagemid vector and transformed into E. coli. VHH phage display is carried out to isolate SARS-CoV-2 antigen-specific clones. After rounds of panning on the antigen of interest, SARS-CoV-2 antigen-specific VHH coding sequence is selected. The identified VHH coding gene is inserted into a yeast expression vector to produce a soluble VHH.
Antiviral advantages of Nbs
Nbs have been explored as antiviral agents in many respiratory illnesses, such as Middle East respiratory syndrome (MERS), influenza type A, and respiratory syncytial virus infections. Recently, a pair of VHHs from a llama immunized with the stabilized prefusion form of the receptor-binding domain (RBD) of the coronavirus spike protein was found to neutralize both MERS and SARS coronaviruses. The VHH directed against the spike RBD bound to the spike with high affinity. This is down to its neutralizing capacity.
Three other VHHs have been found which bind to the RBD of SARS-CoV-2 and inhibit the RBD-ACE2 interaction that is essential for viral entry into the host cell, a finding which is important in view of the current pandemic.
Several researchers have reported the anti-inflammatory capabilities of Nbs too, which could help reduce cytokine production and the risk of a cytokine storm in severe cases of COVID-19.
The ability to deliver Nbs by nebulization allows them to reach the lungs directly to antagonize inflammation and inhibit viral replication. Their fusion to the Fc part of human antibodies of different classes allows them to be easily engineered to counter different viruses.
Approved Nbs
Nbs have already been under study for some time, cutting down on the time needed to bring them to the point of use. For instance, the drug caplacizumab is a bivalent approved Nb, used for thrombotic thrombocytopenic purpura. Another is vobarilizumab, an IL-6R Nb, which is being studied for its use in rheumatoid arthritis. Their safety as antivirals does need to be established, however, since they undergo rapid renal clearance due to the low molecular weight. This could produce renal damage or diminish tissue to below its effective concentration. In short, these issues all require further work.
Diagnostic applications
Nbs are also potentially useful for diagnostic applications. Several kits are now approved for serological diagnosis, targeting the IgM and IgG antibodies against the virus, respectively. However, their lack of specificity and sensitivity make them unacceptable for general screening. Nbs could solve this issue as part of a rapid antigen kit, with high sensitivity and specificity against SARS-CoV-2. In fact, enzyme-linked immunosorbent assays (ELISAs) have already been developed on the basis of Nbs, targeting specific antigens and biomarkers.
Conclusion
The future holds immense promise for Nbs as part of the therapeutic arsenal, as well as for diagnosis, against SARS-CoV-2, and for other new coronaviruses that might emerge in the future.
Read the original article on Medical News.