Scientists at Osaka University, Panasonic Corporation, and Waseda University used scanning electron microscopy (SEM) and X-ray absorption spectroscopy to determine which additives induce crystallization in supercooled aqueous solutions. This work may lead to the development of new energy storage materials based on latent heat.
If you put a bottle of water into the freezer, you will expect to pull out a solid cylinder of ice after a few hours. However, if the water has very few impurities and left undisturbed, it may not be frozen, and instead remain as a supercooled liquid. Be careful, because this state is very unstable, and the water will crystallize quickly if shaken or impurities are added - as many YouTube videos will attest.
Supercooling is a phenomenon in which an aqueous solution maintains its liquid state without solidifying, even though its temperature is below the freezing point. Although many studies have been done on additives that trigger the freezing of supercooling liquids, the details of the mechanism are unknown. One potential application might be latent heat storage materials, which rely on freezing and melting to capture and later release heat, like a reusable freezer pack.
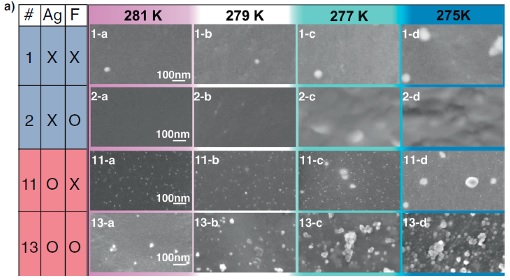
a) HAADF-STEM images of replica films prepared from samples #1, #2, #11, and #13 of tetra-n-butylammonium 3-methylpentanoate (TBA-3MP) aqueous solutions. In samples #11 and #13, silver nanoparticles 5-10 nm across were observed. In sample #13, which included both silver nanoparticles and F- anions, 10-30 nm clusters existed even at 281 K. With further decreases in temperature, the number density of the clusters increased, and then TBA-3MP semiclathrate hydrate crystallized with a small degree of supercooling.
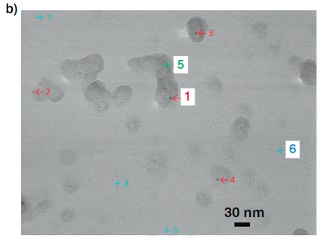
b) SE-STEM image of a different area of the same film as “13-a” in panel a). Clusters of size 10-30 nm envelop a silver nanoparticle (black dots indicated by red arrows).
Now, a team of researchers led by Osaka University has shown that silver nanoparticles are very effective at inducing crystallization in clathrate hydrates. Clathrate hydrates physically look like ice and are composed of hydrogen-bonded water cages with guest molecules inside. “Using SEM with the freeze-fracture replica method, we captured the moment when a nascent cluster enveloped a silver nanoparticle in the aqueous solution of latent heat storage materials,” corresponding author Professor Takeshi Sugahara explains. This occurs because the nanoparticles serve as a “seed,” or nucleation site, for tiny clusters to form. Once this gets started, the remaining solute and water molecules can quickly form additional clusters and then cluster densification leads to the crystallization.
The researchers found that while silver nanoparticles tended to accelerate the formation of these clusters, other metal nanoparticles, such as palladium, gold, and iridium do not promote crystallization. “The supercooling suppression effect obtained in the present study will contribute to achieve the practical use of clathrate hydrates as latent heat storage materials,” Professor Sugahara says. Material design guidelines for enhanced supercooling control, as described in this study, may lead to the application of latent heat storage materials in solar energy and heat recovery technologies with improved efficiency.
Read the original article on Osaka University.