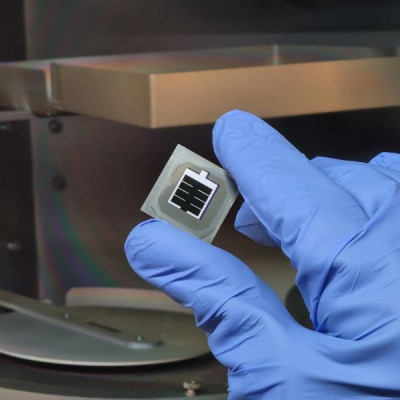
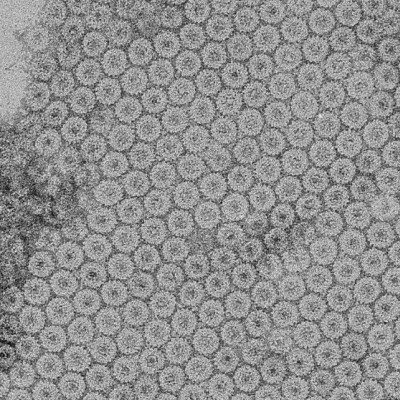
The technology improves the areas efficiency in interface layers between membranes, flow field plates and electrodes in electrolyser cells, these cells can be made both cheaper and increase peak hydrogen production.
The hydrogen production technology that Smoltek is focusing on is called proton exchange membrane (PEM), a process that produces very pure hydrogen and has a great advantage in that it can already handle higher current density and more variables loads than alkaline electrolysis cells.
This means that PEM works well together with intermittent energy sources, such as solar and wind power.
The unique quality of Smoltek’s technology concept is that the catalytic nanoparticles of typically platinum nanoparticles of platinum or iridium oxide can be placed on an optimal nanostructure scaffold for the electrolyser cell, allowing for more and better mass transport of the products that arise.
The group will look to complete a technical proof-of-concept for cell components based on carbon nanofibers and initiate a development collaboration with a large-scale manufacturer of electrolysers.
Ellinor Ehrnberg, Head of Smoltek Innovation, said, “Today, many people talk about the challenge of storing and distributing hydrogen, but few seem to be interested in modernising the production technology.
“Today’s technology is for example using very expensive and noble catalyst materials that do not come into full contact with the membrane, while Smoltek’s technology makes it possible to structure them for optimised contact and thus achieve full effect with a lower amount of expensive particles.
“With Smoltek’s technology, it should be possible to produce two to three times more hydrogen per cell compared to the current technology.
“This is because two to three times more catalyst particles can be in contact with the membrane at the same time, thanks to the optimising of the catalyst layer.”
Read the original article on Hydrogen View.