Immunotherapy has showed great potential in clinical cancer treatment due to systematical activation of antitumor immunity. However, low immunogenicity, or negative feedback on immunotherapy, can greatly hinder the efficacy of current cancer immunotherapy.
In a study published in Advanced Materials, a team of researchers led by YU Haijun from Shanghai Institute of Materia Medica, Chinese Academy of Sciences (CAS), proposed acidity-activatable dynamic nanoparticles to boost ferroptosis and immunogenic death (ICD) of tumor cells for cancer immunotherapy. Ferroptosis is a new type of cell death caused by lipid peroxidation (lip-ROS). The repairment axis of lip-ROS contains glutamate–cystine antiporter for synthesis of intracellular glutathione (System XC-) and Glutathione Peroxidase 4 (GPX4), both of which play important roles to fight against lip-ROS. It was reported those produced lip-ROS can act as “find-me” signals to promote the phagocytosis of antigen-presenting cells, and further activating cytotoxic T lymphocytes to enhance tumor immunotherapy.
The researchers firstly synthesized amphiphilic acid-sensitive block copolymer coupled with photosensitizer (pyrochloric acid, PPA) and phenylboric acid through hydrophobic interaction and π-π conjugation to encapsulate insoluble GPX4 inhibitor (RSL-3). And the prepared nanoparticles could be selectively enriched in tumor tissues through EPR effect. Then the researchers found nanoparticles with external light can induce obvious ICD as well as cytotoxic T lymphocytes which secrete IFN-γ. More importantly, IFN-γ and RSL-3 presented synergistically inhibition on the repairment axis and SystemXC--GPX4, while increased the accumulation of lipid-ROS in tumor cells, thus revealing the interaction between ferroptosis and immunotherapy.
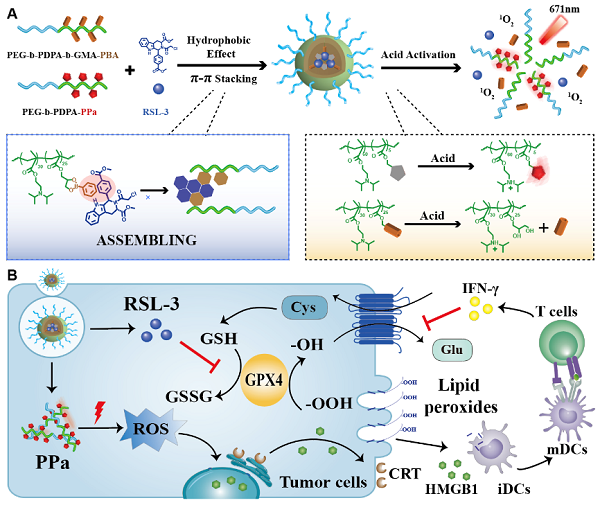
schematic of acid-activated nanoparticles for inducing ferroptosis of tumor cells and enhancing antitumor immunotherapy.
Also, the researchers found that the nanoparticles combined with immune checkpoint therapy (ICB) dramatically reduced the tumor metastasis in mouse breast tumor models. It showed that the nanoparticles reduced the tumor-infiltration of dedifferentiated tumor cells in a manner of ferroptosis, and whose relative frequency were reduced by 4 times after treatment of nanoparticles plus αPDL1. Furthermore, the tumor-infiltration of cytotoxic T lymphocytes in the tumor tissues were increased by 4 times, which effectively inhibited the growth and metastasis of tumor and improved the survival rate of tumor-bearing mice.
Read the original article on Shanghai Institute of Materia Medica (SIMM).