Gastric (stomach) cancer (GC) is one of the most aggressive cancers with a low early diagnosis rate. Current GC therapies are focused on conventional chemotherapy, where chemo drugs act as a potent non-specific intracellular poison to inhibit cell division. They can kill the cancer cells, but at the same time, they are toxic for healthy tissues. Therefore, there is an urgent need for developing more effective treatment methods for GC to overcome its increasing incidence.
In this sense, water-soluble and atomically precise gold nanoclusters (AuNCs) have emerged as a promising material for cancer nanomedicine due to their exceptional features, such as excellent biocompatibility, superior stability, and efficient renal clearance (i.e., they can be removed from the body by the kidney and excreted in urine).
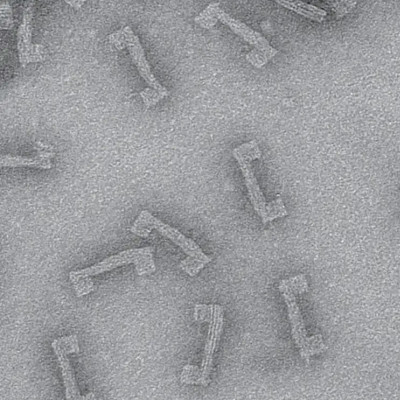
The research group of Professor Hannu Häkkinen took advantage of these unique properties and designed and characterized at a computational level a set of multifunctional AuNCs to be potentially employed as combination therapy in GC treatment. The key to functionalize the AuNCs for applications is to modify the molecular layer covering the metal center of nanocluster. The research is the first paper published in ACS Nanoscience Au (open access).
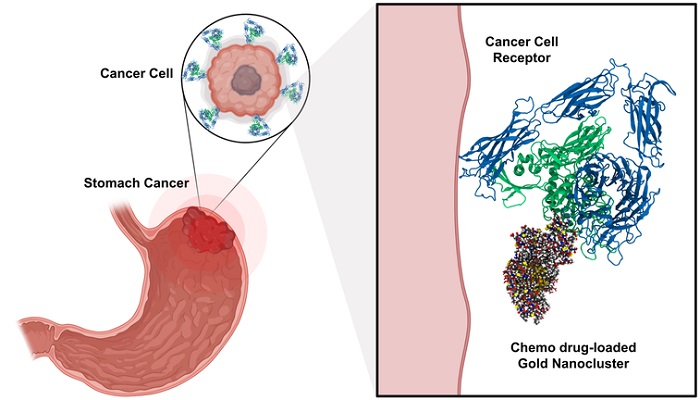
Targeted and chemo drug-loaded gold nanoclusters can recognize and bind to tumor-specific receptors found on the surface of cancer cells. After entering the tumor cell, the chemo drug is released increasing the efficacy of the treatment and reducing toxic effects on surrounding healthy tissue.
The new functionalized AuNCs include a therapeutic component (chemo drug) and hold a targeting action by peptide molecules. Loaded by these molecules, AuNCs can recognize tumor-specific receptors which are overexpressed in cancer cells and act specifically on that site (see Figure).
The researchers were able to elucidate the most promising ligand combination and how the peptide/drug ratio can affect the potential targeting ability of the AuNCs using all-atom molecular dynamics simulations. The most promising features were observed when the peptide is favored over the drug; however, the authors demonstrated that the functionalization must be made case-by-case since the properties of the nanosystem is governed by several factors, including the composition of the AuNCs’ surface and the interactions between the different structural components.
“These computational methods provide efficient screening tools to design optimal nanosystems and allow selecting the best candidates among many options which optimizes resources in the experimental stage that follows” says postdoctoral researcher Francisca Matus who is the lead author of the study.
“This exciting work expands our knowledge and internal collaborations at Nanoscience Center of the University of Jyväskyla to nanomedicine”, comments Professor Hannu Häkkinen, who coordinated the study.
Read the original article on University of Jyväskylä.