Researchers from the Japan Advanced Institute of Science and Technology (JAIST) have developed a new methodology to assess the technical feasibility of storing hydrogen by using carbon nanotubes.
The potential of single-walled carbon nanotubes and other nanostructures as a medium for storing hydrogen has been investigated by the scientific community over recent decades but this technology has so far failed to reach commercial maturity as hydrogen becomes a gas at -252 degrees Celsius, which makes its storage at room temperature using bare carbon nanotubes impossible.
The main issue for this kind of hydrogen storage is that the interaction energies between adsorbed molecules and the nanotube walls are not high enough to store big quantities of hydrogen at room temperature and relatively low pressures. “The interaction between hydrogen and its storage material is simply too weak to persist at room temperature,” the Japanese group stated. “This makes the design of storage materials crucial to achieving the goal of bringing hydrogen energy into daily use.”
The scientists focused their analysis on carbon nanotubes made of silicon carbide, a substrate material that is extensively used in the electric vehicle (EV) industry and the PV sector, which uses silicon carbide in next-generation inverters.
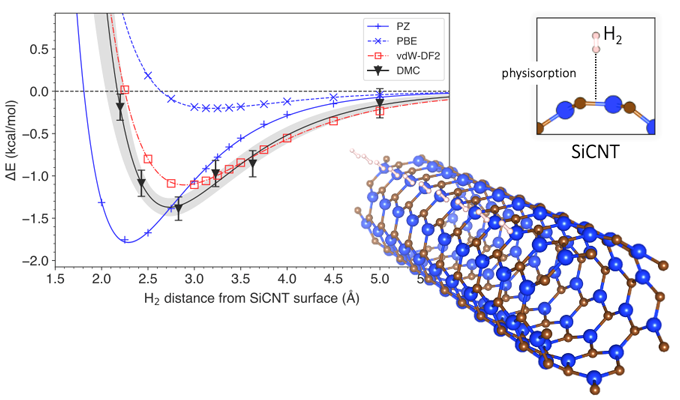
The energy change associated with hydrogen removal from silicon carbide nanotubes.
Through diffusion Monte Carlo (DMC) analysis, the academics created a model that accounts for Van der Waals forces in the simulation. The Van der Waals forces define the attraction of intermolecular forces between molecules. “Most conventional models consider the interactions between hydrogen and silicon-carbide nanotubes as a whole, but the DMC method uses the power of a supercomputer to reconstruct the interaction mechanism faithfully by following the arrangement of individual electrons,” they further explained.
The proposed methodology is claimed to have established how much energy would be necessary to dislodge hydrogen from its storage, and how far away the hydrogen was likely to be from the surface of the silicon-carbide nanotube. The results of the simulation were then compared to those achieved by traditional prediction methods based on the density functional theory (DFT), which calculates the electronic structure of atoms, molecules, and solids.
The researchers found that the DFT analysis without Van der Waals forces wrongly estimated the energy required for hydrogen storage by 4-14%. The analysis that included Van der Waals forces, by contrast, found storage energy was estimated at 9-29%, a percentage range that the scientists defined as “hardly insignificant.”
“Improving prediction reliability for simulations can help accelerate the development of materials for hydrogen fuel storage and lead to a more energy efficient society,” said JAIST researcher Kenta Hongo. “Although the DMC method is computationally expensive, it can be used to clarify the peculiarities, and tendencies of prediction error, of each prediction method.”
The findings of the research can be found in the paper "Importance of Van der Waals Interactions in Hydrogen Adsorption on a Silicon-carbide Nanotube Revisited with vdW-DFT and Quantum Monte Carlo", published in ACS Publications.
Read the original article on pv magazine.