For several years, the group has produced beautiful crystal structures at the nanoscale that range from corals and vases to helices. These structures form spontaneously from various chemicals in a process called self-assembly. Their shape depends on the quantity and type of substances mixed together. The research is aimed at understanding and controlling the process.
There has been some success in that regard. For example, the researchers can choose whether they want to produce a coral or vase, but not where or when the growth starts. “The process still contains a form of chaos. It remains a spontaneous process that we would like to gain more control over,” says group leader Wim Noorduin. Bistervels has now shown that light is highly suitable to achieve this. With a narrow ray of UV light, she can very precisely and selectively influence a chemical reaction at the micrometer scale.
Switch for chemical reaction
The florescent structures the researchers produce arise as a result of a simple chemical reaction. They are composites of two substances: barium carbonate (BaCO3) and silica. As soon as barium carbonate crystals form in the solution, the silicon joins in and precipitates together with the crystals, thus giving rise to the unusual shapes. A tiny bit of CO2 gas in the solution starts this process. If one could ensure that CO2 arises at the exact location and time desired, this would result in an on-off switch for the chemical reaction.

A timelapse with optical microscopy images of the growth of a triangular BaCO3-silica nanocomposite. Using a static triangular UV light pattern (pictured left), the researchers controlled the contour of the nanocomposite in the shape of a triangle. Because the building blocks are generated in the light, the growth of the nanocomposite follows the light pattern. The photo on the right was taken with an electron microscope.
We now have that switch. By illuminating the solution with a UV lamp (similar to that in a sunbed), one of the chemicals in the solution decomposes and forms CO2 at the exact location where the light shines.
Unique microscope
Bistervels quickly saw that her idea worked, but that the standard microscope she wanted to use to make the florescent structures visible did not work well in combination with the UV lamp. She therefore built a special microscope together with technicians Marko Kamp and Hinco Schoenmaker. With this microscope, it is possible to control the UV light very accurately, even remotely from home. One can immediately see the crystals formed through the microscope and, if necessary, adjust the self-assembly process. Fred Brouwer, Professor of Photochemistry at the University of Amsterdam, helped the researchers with his knowledge of light and chemical reactions. “Thanks to these unique collaborations, we were able to combine the strengths of chemists and physicists. I have learned a lot from this,” says Bistervels.
Bistervels demonstrated that with this approach, one can exert a considerable amount of control over the structures that form. She constructed a helix and a coral close to each other, simply by moving the ray of light slightly and making a minor adjustment to the chemical reaction. Furthermore, she demonstrated that a very large number of crystals can be produced next to each other in a pattern. “These experiments are not trivial,” she says. “You need different conditions and a multifaceted control over time and location.”
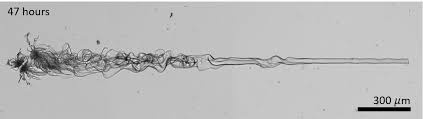
An optical micrograph of a several millimeters long BaCO3-silica nanocomposite. The nanocomposite is grown using a dynamic UV light pattern. By moving the UV light pattern at the correct speed ahead of the growth front, where the building blocks are generated, the researchers can control the growth direction of nanocrystals. It took 47 hours to grow this millimeters-long line of nanocrystals.
The most peculiar experiment was drawing a line, the researchers state. Although that might appear to be nothing spectacular, Bistervels says: “This demonstrates how much control we have. Taming the direction in which the crystals grow is an amazing achievement. You control a process at the nanoscale and see the outcome with the naked eye.”
Controlling biomineralization
The structures are more than just a sight to behold. By learning how they can use light to control the development of the structures, the researchers have acquired important knowledge about self-assembly. “We can apply the methods to manipulate local chemical reactions to similar self-assembling systems. In addition, we see possibilities to use these new methods to gain a better understanding of biomineralization in nature, such as the formation of bone,” says Noorduin.
In another project, the Self-Organizing Matter group has succeeded in converting the crystals into semiconductors. These are vital materials for solar cells, LEDs, and computer chips. Noorduin explains: “If we can produce semiconductors of any desired shape without the need for an expensive and complex cleanroom, then that would offer interesting possibilities. An example is the manufacture of electronic components by means of self-assembly. We are therefore currently investigating how we can control three-dimensional structures, so that we can subsequently produce patterns.”
Read the original article on AMOLF.