Graphene has been touted as a wonder material that can potentially revolutionize the semiconductor industry, owing to its exceptional electron mobility properties. Despite the hype, it appears our civilization is still far from transitioning from the silicon age to the graphene age. The main challenge of using graphene in electronics is the zero-bandgap electronic structure of graphene. This makes it impossible to switch off graphene-based transistors, which limits their application in the semiconductor industry. While it is possible to overcome this limitation by doping or functionalizing the graphene, there is also much interest in the search for new types of 2D carbon allotropes that have exceptional semiconducting properties, such as a proper energy bandgap and high mobility.
Recently, researchers discovered that it is possible to endow many characteristics suitable for a semiconductor to graphene or graphene oxides by creating many holes in its structure. This new type of material is called “holey graphene.” Compared to graphene, γ-graphyne, or graphdiyne, holey graphene not only has the ideal 2D semiconducting properties but also has nonlinear sp bonding and a special π-conjugated structure, which offers promising applications in optoelectronic, energy harvesting, gas separation, catalysis, water remediation, sensor, and energy-related fields.
So far, holey graphene has been produced in laboratories by first synthesizing graphene, then subjecting the graphene to physical, chemical, or hydrothermal treatment to puncture many holes in the structure. However, such a top-down approach for production has its limitations because the size and distribution of the ‘holes’ are uneven and difficult to control.
Led by Associate Director LEE Hyoyoung, researchers from the Center for Integrated Nanostructure Physics (CINAP) within the Institute for Basic Science (IBS), South Korea, developed a bottom-up approach for creating such material. For the first time, the group devised a method to construct topologically 2D carbon material atom by atom.
This new two-dimensional single-crystalline material was dubbed “holey-graphyne” (HGY) by the group. HGY consists of alternately linked between benzene rings and C≡C bonds, comprised of a pattern of six-vertex and highly strained eight-vertex rings and an equal percentage of sp2 and sp hybridized carbon atoms.
“We were inspired by an intriguing molecule, dibenzocyclooctadiyne, which was first synthesized by Sondheimer and co-workers in 1974. In dibenzocyclooctadiyne, two aromatic benzene rings are connected by two bent acetylenic linkages, resulting in a highly strained eight-membered ring. This exciting molecule inspired us to design and synthesize the new carbon allotrope, version of the material, namely holey-graphyne,” said Associate Director Lee.
The research group successfully produced the ultra-thin single-crystalline HGY using 1,3,5-tribromo-2,4,6-triethynylbenzene as the base material. The single atomic layer thin HGY was then synthesized between the interface of two solvent-system consisting of water and dichloromethane. The new HGY displayed a direct bandgap of about 1.1 eV and excellent calculated-carrier mobility, making it suitable as a semiconductor material.
This new discovery not only demonstrates the first synthesis of the ultrathin single crystalline HGY but also introduces a new concept for the design and synthesis of such a new type of 2D carbon allotrope. It is hoped that the future application of HGY in the semiconductor industry will pave the wave for a new generation of electronics beyond the silicon age.
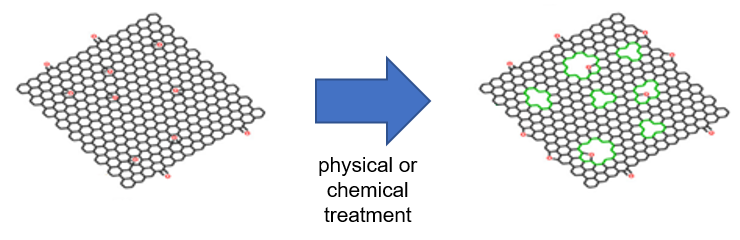
Figure 1. Holey graphene produced using a conventional top-down approach. The size and distribution of holes generated are uneven.
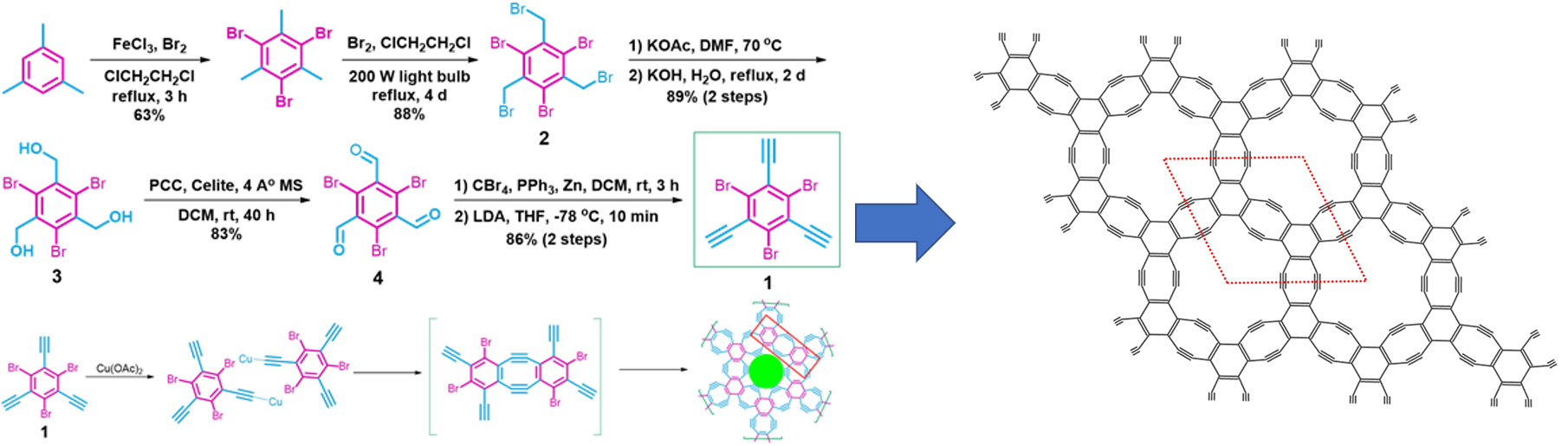
Figure 2. Holey graphyne (HGY) produced using bottom-up synthesis. The molecular structure of HGY is highly consistent with alternately linked benzene rings and C≡C bonds, comprised of six-vertex and highly strained eight-vertex rings and an equal percentage of sp2 and sp hybridized carbon atoms.
Read the original article on Institute for Basic Science (IBS).