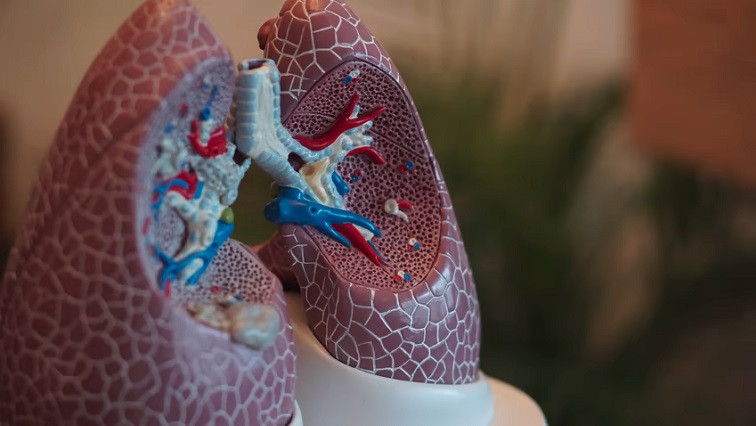
The investigational candidate, Reqorsa, consists of the tumor suppressor gene TUSC2 encapsulated in a positively charged lipid nanoparticle. Because cancer cells generally have a negative electrical charge, Genprex thinks intravenously administered nanoparticles will specifically target the tumor, leading to the expression of the TUSC2 gene and a range of anti-cancer effects.
Genprex wants to find out whether those effects can enhance Keytruda. The phase 1/2 clinical trial is focused on NSCLC patients who progress after treatment with Merck’s checkpoint inhibitor. If Genprex is right, Reqorsa could give those patients a new option by modulating the immune system.
The first part of the phase 1/2 clinical trial will enroll up to 30 patients for dose escalation. Once it has found the maximum tolerated dose of the combination, Genprex plans to enroll around 126 patients to receive either the Reqorsa-Keytruda combination or docetaxel and/or ramucirumab. The phase 2 part of the trial will assess progression-free survival in the two cohorts.
Genprex is aiming to complete the phase 1 part of the trial by the first quarter of 2023. An interim analysis of the efficacy data will happen after 50 events. Genprex expects its existing cash and marketable securities to fund its operation into 2024.
Read the original article on Fierce Pharma.