The invention of the optical microscope in the seventeenth century ushered in a new era of biological research as scientists were able to peer into a previously unknown world of cells and microbes. Another revolution in the life sciences is now underway as nanoscale-imaging techniques are revealing the molecules underlying the processes of life.
“Many biological phenomena are invisible at the micrometre-scale resolution of a conventional microscope,” says Takeshi Fukuma, a professor at the World Premier International Research Center Initiative (WPI) Nano Life Science Institute at Kanazawa University in Japan. “Since all the functions of cells are realized through the organization and interactions of biological molecules, we want to be able to visualize them directly.”
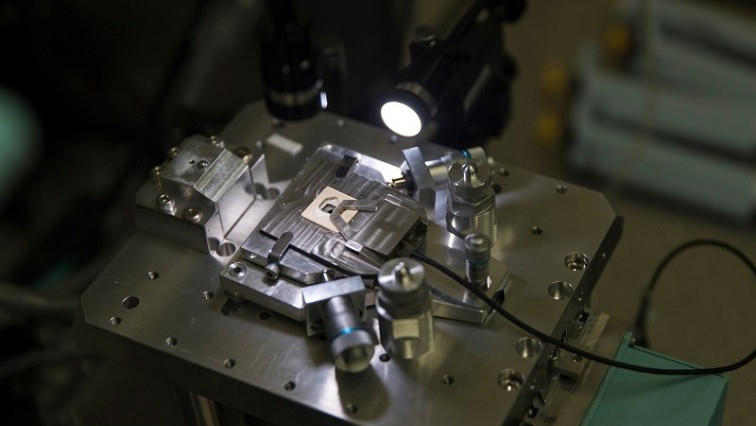
One of the most exciting of these nano-imaging methods is atomic force microscopy (AFM). Invented as a way to image the surfaces of materials, AFM has recently found an application in biological imaging. Its advantages include an ability to image living cells and the fact that it is a direct imaging technique that doesn’t require labeling molecules. By extending the basic technique in two different directions, two groups at Kanazawa University are at the vanguard of applying AFM to image biological systems on a nanoscale.
Endoscopy on cells
Fukuma’s team has developed an AFM probe that has a nanoscale, needle-like tip, which enables it to investigate the interior of cells. They have dubbed their technique ‘nanoendoscopy AFM’ because the tip acts like an endoscope probe, imaging the inside of cells in three dimensions as it is inserted. The tip’s tiny size ensures minimal damage is done to living cells.
“Conventional AFM scans a surface or interface to provide a two-dimensional height image,” says Fukuma. “But in nanoendoscopy AFM, we insert a very sharp, needle-like probe into a 3D object and use it to perform 3D mapping of the sample.”
Nanoendoscopy AFM has two key advantages over other nano-imaging techniques, such as cryogenic electron microscopy and super-resolution fluorescent microscopy, Fukuma notes. One is its high spatial resolution. “We can achieve a sub-ten-nanometre resolution inside living cells, which is impossible with any other technique,” he says.
Another major advantage is the ability to use the AFM tip to measure the elasticity and viscosity of structures. “We can detect the quantitative mechanical parameters of the intracellular components,” says Fukuma.
The team has used this second advantage to make a surprising discovery about cancer cells. Many studies have reported that cells become softer when they become malignant. “This is reasonable because it allows them to move easier, which will enhance cancer’s ability to spread to other sites in the body,” explains Fukuma. “However, we found that the cell nucleus actually becomes stiffer when cells become malignant. While this is very unexpected, it also makes sense, because when cells migrate through a small channel, they are subject to high pressures and they need to protect the important content within the nucleus.”
Fukuma is planning to enhance the technique in various ways, including increasing its spatial resolution and achieving faster imaging. He also wants to improve the modelling of the mechanical properties of living cells, so as to obtain more accurate measurements of mechanical parameters.
Molecular movies
A team led by Noriyuki Kodera, a professor at the WPI Nano Life Science Institute at Kanazawa University, is using a version of AFM that has been extended in another direction. Kodera’s former supervisor, Toshio Ando, a distinguished professor at the institute, developed high-speed AFM, which enables molecular-scale-resolution movies to be obtained rather than static images.
Prior to high-speed AFM, researchers had to choose between a technique such as X-ray crystallography or electron microscopy that provides static information at high resolution or use methods such as fluorescence microscopy that gives dynamic information but at low resolution. High-speed AFM now allows researchers to obtain both high resolution and dynamic information at the same time. “High-speed AFM allows researchers to have their cake and eat it — we can explore the dynamics of nanostructures,” says Kodera. “This kind of information can’t be obtained using other techniques.”
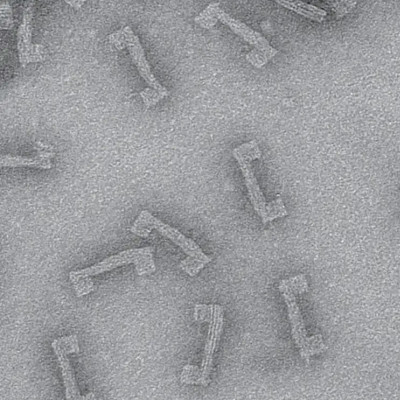
Kodera’s team has used high-speed AFM to explore a neglected group of proteins known as intrinsically disordered proteins. Our limited knowledge of these proteins belies their importance — they make up more than 40% of proteins in eukaryotic cells. However, their writhing, tangled structures make them next to impossible to study using conventional analysis techniques such as electron microscopy, X-ray scattering and nuclear magnetic resonance. But as high-speed AFM can rapidly image at a nanoscale resolution, it is ideally suited for studying intrinsically disordered proteins.
Using high-speed AFM, Kodera’s team looked at intrinsically disordered proteins from various sources, including viruses and yeast. They discovered some regions in these proteins were always folded and others were constantly disordered, but they also spotted some regions that alternated between ordered and disordered states, observing a structural transition between them. “Previously, many researchers believed that intrinsically disordered protein are always disordered, but we have shown for the first time that some regions in them can switch between order and disorder,” says Kodera.
World leader in biological AFM
The team was also able to estimate the number of amino acids in the constantly disordered regions of intrinsically disordered proteins, allowing them to create a rough sketch of the structure of these important proteins.
Kodera and his co-workers are now working on making high-speed AFM even faster. “Our current system captures about ten frames per second, but we’re aiming to make it ten times faster,” says Kodera. “That would make it even more useful and enable us to look at much wider range of biological processes and systems.”
The exciting work that Fukuma and Kodera are doing in the area of AFM is revealing the potential that this powerful technique has for examining the processes of life at a molecular scale, throwing open the uncharted world of nanobiology. Together, they are positioning Kanazawa University as a world leader in the application of AFM to biological systems.
Read the original article on Nature.