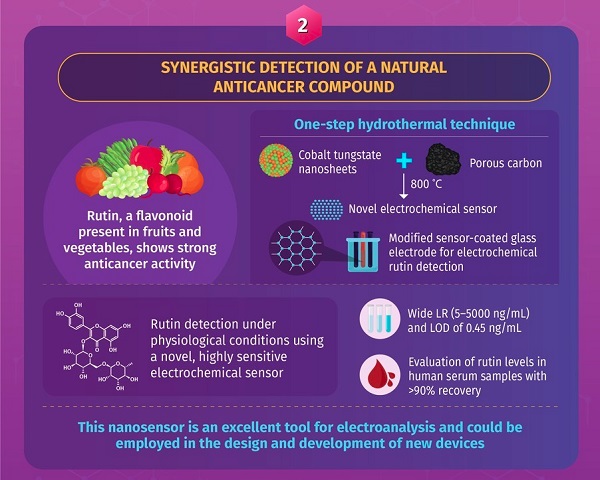
The first study, first made available online on September 16, 2021, describes the development of a nanosensor that allows highly sensitive electrochemical evaluation of rutin, an important anticancer compound. Most previous methods of rutin detection have only been effective under acidic conditions. However, they cannot be applied for rutin evaluation in clinical samples (non-acidic), which is necessary for drug monitoring and individualized drug delivery plans. A team of researchers solved this problem by combining 3D porous carbon (PC) and cobalt tungstate (CoWO4) nanosheets to obtain a nanosensor that detects rutin in clinical samples. They modified glass electrodes with this nanosensor and tested its efficacy in the electrochemical detection of rutin in human serum samples. They found that this nanodetector offered high sensitivity and could detect rutin concentrations as low as 0.45 ng/mL. Hence, the PC/CoWO4-based sensor showed potential as an excellent tool for electroanalysis and can be used for developing new laboratory devices for faster clinical decision-making.
The power of nanotechnology-based for biochemical detection.
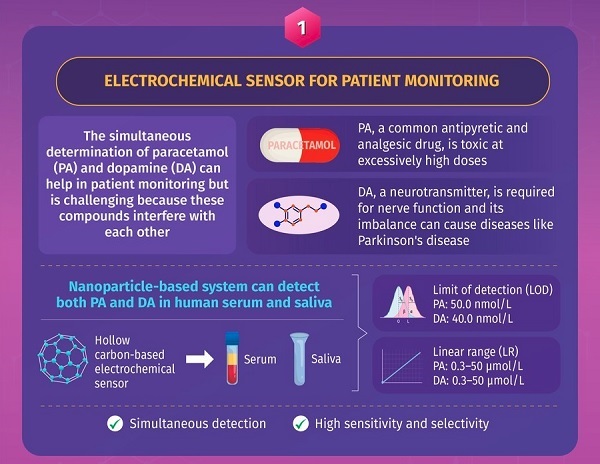
Like rutin, paracetamol, a common fever- and pain-reducing agent, also needs to be evaluated during treatment. Although the simultaneous monitoring of paracetamol and dopamine, a neurotransmitter and biomarker, can help assess a patient's status, it is challenging because these two biomolecules interfere with each other. Addressing this problem, the second study, first made available online on September 1, 2021, focused on the development of nanoparticles for the simultaneous evaluation of these two compounds in clinical samples. In this study, researchers from Fujian, China developed double-shelled spherical nanoparticles and tested their efficacy for paracetamol and dopamine detection in human serum and saliva. Owing to the intelligent design and the presence of double shells, the nanoparticles had several advantages, such as high conductivity and long-time durability. Using these nanoparticles, dopamine and paracetamol levels as low as 40 and 50 nmol/L, respectively, could be detected with high sensitivity and reliability from clinical samples.
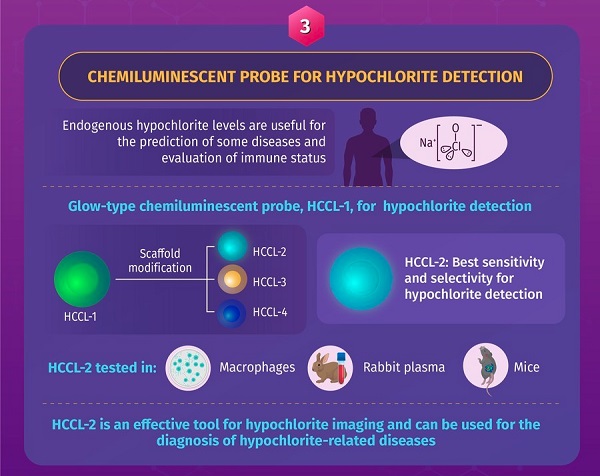
The final study, which reports the design of a fluorescent probe for the detection of the biomarker hypochlorite, a chemical that generates atherogenic lipoprotein granules that may induce heart disease, neurodegenerative diseases, arthritis, and even cancer, was first made available online on October 9, 2021. The researchers used an intricate process to design a glowing, fluorescent hypochlorite probe called hypochlorite chemiluminescence probe 1 (HCCL-1). They then modified this probe using three different methods to design three new probes: HCCL-2, HCCL-3, and HCCL-4, and found that HCCL-2 presented high photon emission. In the presence of hypochlorite, HCCL-2 showed high sensitivity and selectivity for hypochlorite monitoring both in vitro and in vivo. The developed probes can help us detect small molecules easily and provide a powerful method for analyzing and detecting diseases involving hypochlorite.
Together, these highly encouraging advances pave the way for the design of new and advanced nanosensors for chemical detection.
Read the original article on PR Newswire.







