Specific catalysts to clean water
Arsenic is a famously toxic element and long-term exposure to even trace amounts can lead to debilitating and potentially fatal diseases including skin cancer, lung cancer, keratosis, and neurological disorders. 100-200 million people globally are believed to be exposed to arsenic through contaminated groundwater drinking supplies. Water multi-step treatments to remove arsenic already exist, using filtration (adsorption), but this process requires pre-treatment (oxidation) to be efficient. A team of researchers from Imperial College London led by Prof. Weiss has been working with Diamond’s B18 beamline on a new material combining photocatalytic oxidation with adsorption, which allows a one-step treatment of contaminated water. Their findings were recently published in Results in Surfaces and Interfaces. The high versality of B18 allowed them to perform X-ray Absorption Spectroscopy (XAS) on their samples and compare the results with their predictive models.
Spectroscopic observations to confirm a predictive model
Arsenic can be found in several forms in water including arsenite and arsenate. Arsenite (As(III)) ions are difficult to remove from contaminated water using conventional treatments such as adsorption or coagulation-flocculation due to its neutral charge (H3AsO3). In contrast, arsenate (As(V)) ions (HAsO42- and H2AsO4-) are both (i) more easily adsorbed and (ii) less toxic than H3AsO3. Consequently, the treatment of As(III)-contaminated water benefits from the oxidation of As(III) to As(V) prior to its removal via adsorption. Oxidation of As(III) can be achieved using heterogeneous photocatalysts, such as TiO2, and ultraviolet (UV) radiation. However, other materials such as iron oxides (Fe2O3) are more efficient for adsorption. Therefore multiple-step treatment plants are currently used but their design and maintenance is challenging. This difficulty may be overcome by incorporating photocatalytic and adsorption capabilities into a single material.
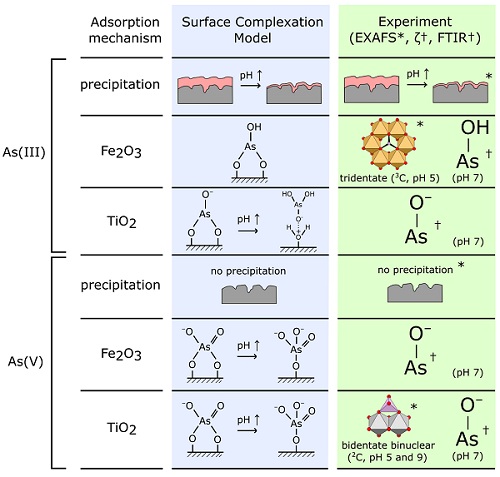
The authors combined spectroscopic data obtained at Diamond Light Source (EXAFS) with FTIR and zeta potential measurements at Imperial College London to develop a picture of the dominant surface complexes formed when arsenic is adsorbed by TiO2/Fe2O3 composite nanomaterials. This was used to evaluate and verify the speciation of adsorbed arsenic predicted by the authors’ previous Surface Complexation Model (SCM), a theoretical model that is able to predict changes in adsorption as a function of environmental conditions. This model had been developed using surface complexes chosen based on data for pure titania and pure iron oxides, with experimental evidence for arsenic speciation on composite TiO2/Fe2O3 not being available prior to the Diamond Lightsource study.
Arrows indicate where the pH is increased from 5 to 9. Asterixes (*) and daggers † indicate evidence gained by EXAFS and FTIR/zeta potential analysis respectively.
Previous studies by the same group showed that composite materials combining the excellent photocatalytic capabilities of TiO2 with the high As(V) adsorption capacities of iron oxides (Fe2O3) were good candidates for efficient decontamination. The authors subsequently developed a surface complexation model (SCM) to predict changes in the amount of arsenic adsorbed and its speciation as a function of experimental variables such as pH. However, the authors wanted to verify that the structures of the adsorbed arsenic surface complexes chosen for the model were realistic, so they used spectroscopic techniques. X-ray Absorption Spectroscopy (XAS) played a key role thanks to its element selectivity, its capability to discriminate As oxidation state (XANES) and to identify the structure of the As complexes adsorbed on the TiO2/Fe2O3 surface (EXAFS).
Dr Bullen, first author of the publication, explains:
We wanted to better understand the nature of arsenic adsorbed onto this composite TiO2/Fe2O3 nanomaterial; to understand whether arsenic binds in the same way as onto pure TiO2 and pure Fe2O3 minerals. X-ray Absorption Spectroscopy is the perfect way to do this, with EXAFS revealing information about the local environment of arsenic atoms, e.g. how many covalent bonds arsenic forms with the TiO2/Fe2O3 surface. The beamtime grant we received allowed us to realise these experiments at Diamond Light Source.
From a model to an application
The team used B18 to realise their experiments. At the beamline, they were able to record EXAFS spectra of solid samples to determine how many covalent bonds arsenic forms with the surface of the materials, and XANES spectra of aqueous suspensions to measure photooxidation kinetics.
Dr Bullen continues:
These experiments allowed us to directly investigate the structure of the surface complexes formed when arsenic binds to our TiO2/Fe2O3 nanomaterial. We were able to conclude that the surface complexes we had chosen for our predictive surface complexation model, using pure titania and pure iron oxide reference materials, were appropriate. However, we also saw some tridentate As-Fe2O3 bonding in the EXAFS data that wasn’t included within our earlier model. We were also able to confirm surface precipitation of As(III) at low pH, which our model had predicted.
The team hopes that the new material might be incorporated into a filtration column for everyday use in affected households, as this cheap and efficient technology could improve the quality of water for millions of people. The researchers already have a patent for this material for arsenic decontamination.
Read the original article on Diamond Light Source.







