“If you are interested in working with blood stem cells in Sweden, this is the place to be.” So says Martin Hjort, a researcher in chemical biology, who is focusing on reprogramming cells.
Martin Hjort has one foot in the cleanroom lab at NanoLund at the Department of Physics, where he makes nanotube membranes small enough for cells to stick to them like lint on a Velcro strip.
He keeps the other foot in the Faculty of Medicine’s lab, where he sneaks DNA and RNA into cells to reprogram them with new and better properties.
“I don’t quite fit in there,” says Martin Hjort.
Martin Hjort is a civil engineer and physicist. He has worked on areas ranging from semiconductor physics and “ultra-high vacuums etc.” to advanced biology. This makes it necessary to team up with medics.
“I can ask questions without losing face. I have to work with others on the applications of my research,” says Martin Hjort and names his colleagues Jonas Larsson and Ludwig Schmiderer.
Umbilical cords demand speed
As to why he began focusing on blood stem cells, the answer is simple.
“I like blood. It is easy to get from patients. A lot easier than a brain biopsy, for example. Blood is also more interesting, as it involves the whole body on a systemic level. I am interested in specifically blood stem cells because they are a difficult, but very important, type of cell. It is the only type of stem cell that is routinely used in clinical practice – for bone marrow transplantation,” says Martin Hjort.
How do you go about getting them? The blood stem cells he uses in his research come from fresh umbilical cords from maternity wards around Skåne. Blood stem cells are sensitive, so it is essential to be quick. Old blood does not provide many stem cells. The geographical proximity between the University and the hospital in Lund plays a big role.
“Technical advancement and medical science must go hand in hand,” says Martin Hjort.
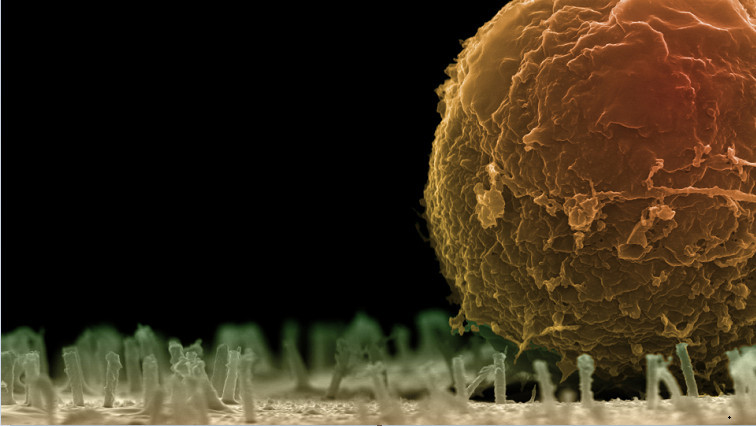
He improves the cells by inserting different molecules through small nanotubes, which create a pathway into the interior of the cells. At the bottom of a glass tube 5 mm in diameter is a membrane of tiny nanotubes 100 nanometres thick – one thousandth of a strand of hair. Martin Hjort produces these in the nano lab using plastic and aluminium oxide in an almost ridiculously simple process.
“There are only three or four steps to it. You take a water filter membrane, add aluminium oxide to cover the surfaces, even inside the pores, and then remove the top layer of aluminium and then some of the plastic layer. Aluminium oxide is stable and commonly used in semiconductor technology.”
The blood stem cells then adhere to this microscopic Velcro surface.
“The nanotubes act like traps for the cells. The cell, in turn, doesn’t even detect the insertion of the tubes. The cell membrane is essentially intact, except where the nanotube was inserted. This method is without a doubt the kindest to the cells, and keeps them the happiest. The cell is a living entity that can get stressed and deteriorate. A bit like humans,” says Martin Hjort.
Rapid technological development
Getting cells that perform as well as possible and are not functionally impaired, is key. But how and why the method seems to be so gentle is beyond Martin Hjort’s area of expertise.
“Instead of thinking about the exact mechanism behind it, I have chosen to focus on finding things that work,” says Martin Hjort.
The pace of technological development in electronics is extremely fast and it can be difficult to find purely technological innovations that deliver growth precisely because of the fierce competition. Ten years have passed since Lund, Stanford and Berkeley simultaneously came up with different methods to use nanotubes to insert things into cells.
“We need the technological development as well, and Lund is in a good position in that regard. But there is more potential in the interdisciplinary approach, where the technological and the medical aspects together allow one plus one to equal three. But I may not have the same structured approach to research as a medical doctor. I might have a more improvisational and curiosity-driven approach to it,” says Martin Hjort.
There is a plethora of diseases that can be corrected with the nanotube-assisted editing of cells. Martin Hjort says there is currently a lot of interest in sickle cell anaemia – a collective term for genetic abnormalities in the shape of the haemoglobin in our blood.
“Of course, when we’re talking about taking the cell out of the patient, inserting the CRISPR (popularly known as the genetic scissors) and using it to say to the cell: now fix the patient’s own cells – when it’s time to put the cells back into the patient, you have to be sure that you have really only cut exactly where you should. Otherwise, you risk creating something that could become malignant instead. With the nanotubes, we can do this much more gently than has been possible in the past, and therefore retain more cells that perform better.”
That said, he believes strongly in the method. He, Jonas Larsson and Ludwig Schmiderer have together already won an innovation prize for their work on improving blood stem cells using nanotubes without it being apparent that they have been modified. Interest in materials science has broadened to biological aspects. Methods should ideally be scalable from the outset.
“There is still a lot to do. People don’t want to get sick. Or have to face harsh treatments,” says Martin Hjort.
Read the original article on Lund University.