Nanovesicles are a popular way to deliver therapeutics, but mammalian-based nanoparticles and synthetic lipid nanoparticles often have difficulty reaching the brain, the lungs, the retinas, and other tissues. They also come with inherent risks. These include low stability, lack of convenient tropisms, and premature clearance or degradation.
To overcome the challenges that arise with conventional nanoparticles, AGS Therapeutics develops microalgal extracellular vesicles (MEVs). According to AGS Therapeutics, MEVs offer a way to overcome some of the body’s key natural barriers. Moreover, the company asserts that MEVs are uniform, easy-to-manufacture delivery vehicles for RNA, DNA, proteins, and small molecules.
AGS Therapeutics is finalizing its choice of specific targets for its initial internal pipeline. Candidates are likely to include vaccines and immunomodulatory agents that can reach the gut, the spleen, the eyes, and the respiratory system.
MEVs bypass biological barriers
Early work suggests that MEVs can survive passage through the stomach. “When you ingest them, they pass through the stomach, get to the intestine, and then enter the body,” says Manuel Vega, PhD, co-founder and CEO of AGS Therapeutics. “That’s not the case for most nanovesicles in development.”
Unlike oral medications that enter the bloodstream from the intestines, MEVs go straight to the “heart of the immune system,” Vega notes. Specifically, they enter the gut-associated lymphoid tissue and then proceed to the spleen.
“We can get there directly,” Vega stresses. “That’s unique.” MEVs, then, can open a new delivery path. By following this path to hard-to-reach tissues, vaccines and immunomodulators put themselves in a better position to fight cancer and other diseases more effectively.
In addition, if MEVs need to reach the brain, they can be administered intranasally. Because a projection of the brain involved in olfaction extends to the base of the nose, MEVs administered intranasally can travel to the brain through the olfactory epithelium, bypassing the blood-brain barrier.
This delivery mechanism “was completely unexpected,” Vega remarks. If it works in clinical studies, it might interest the many drug developers that have found the blood-brain barrier to be a formidable obstacle.
Yet another option for MEV delivery is ocular administration. “We can reach the choroid-retina by eye-drop instillation,” Vega notes.
“MEVs are produced naturally by unicellular microalgae, so they are ‘natural’ rather than synthetic,” Vega says. “By culturing the microalgae, we can produce really large amounts of vesicles through simple manufacturing techniques. These techniques are scalable and offer an easy pathway to GMP compliance.”
A conceptual shift
AGS Therapeutics was founded serendipitously in Paris in 2020, soon after Vega, a biology researcher turned entrepreneur and industry consultant, had crossed paths with Lionel Navarro, PhD, a research director at the French National Research Council (CNRS). “Navarro had originated a foundational patent [for a technology based on Chlorella vulgaris],” Vega recalls. “The technology was amazing.”
C. vulgaris is a two-billion-year-old single-cell alga that has been used for decades as a food supplement. According to Navarro’s patented technology, C. vulgaris is a natural and efficient producer of extracellular vesicles that can be loaded with specific short interfering RNAs. Moreover, as Vega emphasizes, the MEVs are nontoxic in vivo.
“We discussed the technology’s potential with the CNRS,” Vega says, “and we licensed the patent.” Also, Navarro became one of the company’s co-founders. Since then, AGS Therapeutics has been expanding its intellectual property portfolio. Over the past year, the company has prepared or filed approximately one patent every month and a half.
The MEV approach at AGS Therapeutics became possible through a conceptual shift. “We decided to think outside the box,” Vega relates. “First, as biologists, we were motivated more by biological nanoparticles rather than by synthetic or chemical nanoparticles. Second, we didn’t want to use mammalian extracellular vesicles. So many people were already doing that. Moreover, mammalian extracellular vesicles have many obstacles to overcome before they can serve as therapeutic vehicles. So, we decided to try something else.”
That “something else” was selected to ensure that the technology would possess what the company deemed desirable characteristics. They include high biocompatibility, easy manufacturing, the requirement for fresh water as opposed to sea water, the potential to be genetically engineered and tailored, and the ability to be scaled for industrial-scale applications.
“The criteria are met by MEVs.” Vega points out. “We can grow the microalgae with only fresh water, light, and minerals, and the MEVs are easy to manufacture and highly homogenous.”
“There is no intrinsic risk that MEVs carry viruses that are potentially dangerous for humans,” Vega continues, “and regardless of what is put inside the vesicles, their exteriors remain the same.” He concludes that the combination of attributes possessed by MEVs suggests that they can be used in a variety of commercial applications.
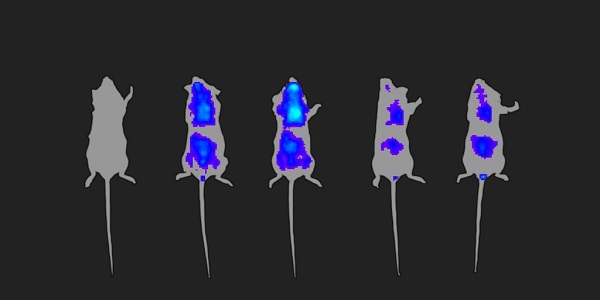
AGS Therapeutics is developing its microalgal extracellular vesicles (MEVs) so that they may serve as drug delivery vehicles. In this image, we see in vivo expression of luciferase mRNA in the lungs and intestine of a mouse 0, 6, 30, 48, and 54 hours after a single intratracheal aerosol dose of mRNA-loaded MEVs. (Expression is also observed in the naso-buccal cavity—not unusual after aerosol administration.) Luminescence intensity reflects the level of expression of the luciferase protein. Notice that peak expression occurs about 30 hours after administration.
A competitive industry
AGS Therapeutics is an early-stage firm that competes in a market that has seen several billion-dollar deals over the past few years. In this market, other companies tend to focus on lipid nanoparticles or natural mammalian extracellular vesicles. Companies pursuing the latter option face daunting challenges with large-scale manufacturing and the relatively limited biodistribution of reachable targets.
The challenges for AGS Therapeutics involve moving the technology into preclinical and then clinical stages and growing the company. At present, it is recruiting in-house staff and conducting much of its development work with external entities. With proof-of-concept and validation data generated, the “real challenge,” Vega says, “is getting properly financed so that we can move the technology to the clinic.”
Funding is a challenge for virtually all young companies, and in that regard, AGS Therapeutics is no different. Yet, with the company’s highly differentiated technology, Vega seems optimistic about attracting early-stage investors. He is putting together a Series A financing round with the goal of attracting venture capital investors in the United States and Europe.
Because AGS Therapeutics has a unique microalgal approach, the company must produce its own biological materials. From the beginning, Vega and Navarro (and the company’s other co-founders, Lila Drittanti, PhD, and Marie-Hélène Leopold) knew they needed two platforms—one focused on the technology and pipeline products, and the other focused on manufacturing. Those platforms are employed by sister companies.
“The manufacturing platform, which is employed by AGS Manufacturing, allows us to optimize the processes for production, purification, and characterization,” Vega elaborates, “and it provides the biological material we use for discovery, preclinical and clinical studies.” He adds, “The next step is to move both platforms to preclinical and clinical development.”
Read the original article on Genetic Engineering and Biotechnology News.