Electrolysis of water using renewable resources is a promising technology for hydrogen production, with a growing interest worldwide. This is because hydrogen is seen as a promising alternative to address the increasing concerns over carbon emissions from fossil fuels that can cause climate change. However, hydrogen production via water electrolysis is primarily limited by the sluggish kinetics of an associated process known as the oxygen evolution reaction (OER). Precious metal based electrocatalysts are often used in the OER process to improve its poor efficiency. A pioneering class of catalytic materials known as heterogeneous ferromagnetic single-atom spin catalysts (SASCs), when used together with an applied magnetic field has great potential to accelerate this chemical reaction. However, the design of such efficient and robust SASCs for the OER process is still recognised as a major challenge.
The research team led by Associate Professor LU Jiong, from the Department of Chemistry, NUS developed a general and scalable approach for the synthesis of a series of SASCs with tunable high loading via a hydrothermal approach. This allows the introduction of different molecular precursors including metal salts (M2+), ammonium molybdate tetrahydrate and thioacetamide in a single processing step. The major innovation lies in the creation of operando acidic conditions during synthesis that can suppress the aggregation into metal nanoparticles by the dopant. Using this approach, the dopant is able to disperse itself as single atoms evenly on the material to achieve high-density substitution, which is important for the proper formation of the spin catalyst and to provide the ferromagnetic effect. This is a collaboration with Professor Xin LUO from Sun Yat-sen University, China, Dr Shibo XI from Institute of Sustainability for Chemicals, Energy and Environment, Singapore and Professor Cheng-Hao CHUANG from Tamkang University, Taiwan.
This research breakthrough was published in the journal Nature Nanotechnology.
The researchers used molybdenum disulfide (MoS2) as the host material and found that the doping ratio can be adjusted by up to 20:100 for the various types of M1/MoS2 SASCs (M1=Mn, Fe, Ni, Co). In general, higher doping density reduces the average spacing between adjacent magnetic dopant sites and increases the long-range magnetic order.
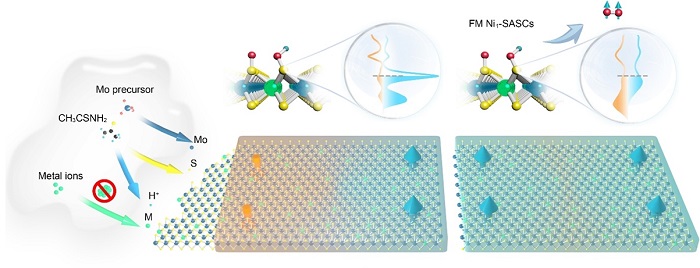
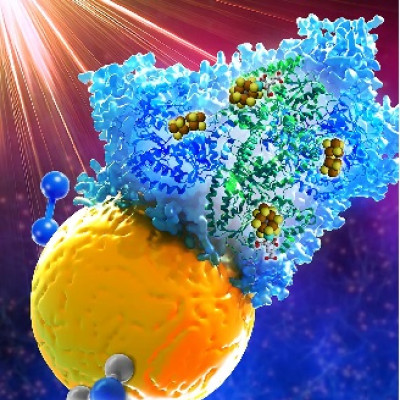
(Left) Illustration of the hydrothermal synthesis of M1/MoS2 single-atom spin catalysts (SASCs) under acidic conditions and (right) the giant magnetic field enhancement of ferromagnetic SASCs used to improve the efficiency of the water splitting reaction.
This research work demonstrates the conceptual design and synthesis of a new class of robust ferromagnetic SASCs with giant magnetic field enhancement (MFE), which can be applied to accelerate both water and saline water electrolysis under commonly available magnetic fields. Although MFE effect on chemical reactions has been exploited in homogenous spin catalysis systems as well as in bulk solid catalysts (e.g. mixed oxides and metal), the design of advanced heterogeneous spin catalysts with atomically precise active sites to boost reaction kinetics and to probe its mechanistic insights remain challenging. This is because one must tackle multiple complexities, including the ability to engineer well-defined active sites with strong short-range quantum spin exchange interaction (QSEI) within individual sites (local magnetism) and long-range ferromagnetic ordering among neighbouring sites (global magnetism).
The major novelty of this work goes beyond the synthesis aspect. Joint experimental and theoretical studies show that all the SASCs developed by the researchers exhibit interatomic QSEI to induce local magnetic moments with spin density delocalised over adjacent sulphur atoms via strong p-d orbital hybridization. In their experiments involving the use of the Ni1/MoS2 SASC, when a mild magnetic field of around 0.5 tesla (commonly accessible magnetic fields from permanent magnets) is applied, it yields a dramatic enhancement of OER magnetocurrent by around 3,000%. It also demonstrates excellent reactivity and stability in both seawater and pure water splitting cells when compared to commercial iridium dioxide (IrO2) catalyst.
Prof Lu said, “This finding reveals that ferromagnetic SASCs provide a powerful magnetoelectric effect to accelerate water electrolysis. The approach offers unprecedented opportunities for the design of non-precious metal-based ferromagnetic SASCs for both water and saline water electrolyser technologies.”
“We plan to work on large-scale synthesis of ferromagnetic single-atom spin catalysts combined with magnetic fields to develop solutions that can reduce the cost and improve the efficiency of green hydrogen production from the electrolytic splitting of water and seawater,” added Prof Lu.
Read the original article on National University of Singapore (NUS) .