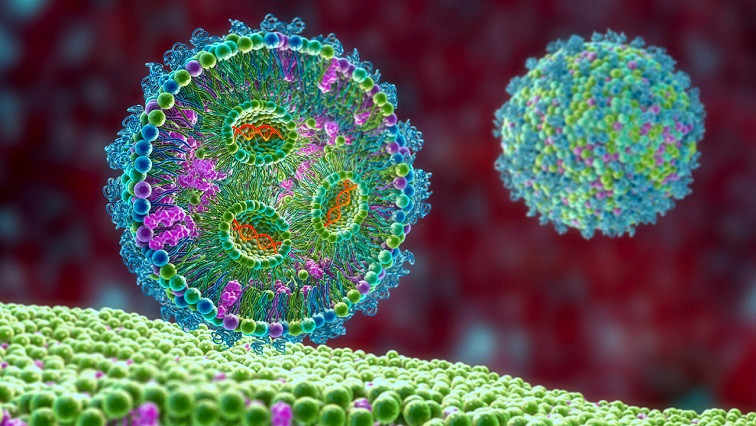
LNPs had a breakout role delivering the cargo in messenger RNA–based COVID-19 vaccines. But Hopewell CEO Louis Brenner says the firm’s founding scientist has been in the business a lot longer, and the “science has been maturing in academia and going into the company for more than a decade.”
Hopewell’s founder and chief technological officer, Tufts University’s Qiaobing Xu, got his start in LNPs when he was a postdoctoral researcher under biochemist Robert Langer at the Massachusetts Institute of Technology in the late 2000s. Recently, Xu’s team at Tufts devised LNPs that can ferry nucleic acid payloads to the lungs and brain in mice, discoveries that are foundational to Hopewell’s platform.
Research veterans agree that although the field has come a long way, more work is needed to sharpen LNPs’ effectiveness as tissue- and cell-specific carriers. Scientists still don’t fully understand why tweaking the LNPs’ charge, head or tail groups, or chemistry cause them to accumulate in certain organs, says Cecilia Leal, a materials scientist at the University of Illinois Urbana-Champaign, so researchers should solve these fundamental questions in parallel. Any design rules that scientists have for LNPs are more empirical than universal, she says.
Another open question is whether LNPs for nonvaccine applications can avoid triggering an immunogenic response, says Gaurav Sahay of Oregon State University.Outside the liver, LNPs still cannot target specific tissues without some spillover to other areas in the body, adds biochemist Pieter Cullis of the University of British Columbia. He suspects the solution is to incorporate additional safeguards in the cargo itself so it activates only when it is in the right location. To have just the LNP doing the hard work of achieving tissue selectivity—“it’s a very difficult proposition,” he says.
Read the original article on American Chemical Society.