When Emiliano Cortés goes hunting for sunlight, he doesn't use gigantic mirrors or sprawling solar farms. Quite the contrary, the professor of experimental physics and energy conversion at LMU dives into the nanocosmos. "Where the high-energy particles of sunlight, the photons, meet atomic structures is where our research begins," Cortés says. "We are working on material solutions to capture and use solar energy more efficiently." His findings have great potential as they enable novel solar cells and photocatalysts. The industry has high hopes for the latter because they can make light energy accessible for chemical reactions – bypassing the need to generate electricity. But there is one major challenge to using sunlight, which solar cells also have to contend with, Cortés knows: "Sunlight arrives on Earth 'diluted,' so the energy per area is comparatively low." Solar panels compensate for this by covering large areas.
Cortés, however, is approaching the problem from the other direction, so to speak: With his team at LMU's Nano-Institute, which is funded, among others, by the e-conversion cluster of excellence, Solar Technologies go Hybrid (an initiative of the Bayerisches Staatsministerium für Wissenschaft und Kunst) and the European Research Council, he is developing so-called plasmonic nanostructures that can be used to concentrate solar energy. In a recent publication in the journal Nature Catalysis, Cortés, together with Dr. Matías Herran, now at Fritz Haber Institute, Berlin, and cooperation partners from the Free University of Berlin and the University of Hamburg, present a two-dimensional supercrystal that generates hydrogen from formic acid with the help of sunlight. "The material is so outstanding, in fact, that it holds the world record for producing hydrogen using sunlight," Cortés points out. This is good news for the production of both photocatalysts and hydrogen as an energy carrier since they play an important role in a successful energy transition.
Concentrating solar energy with miniature magnets
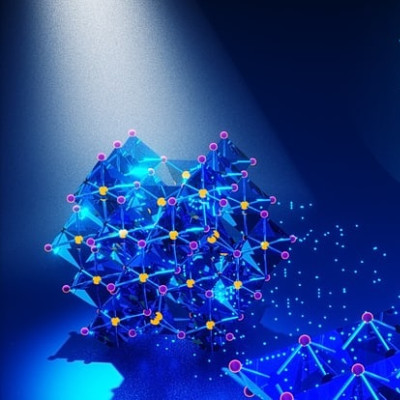
For their supercrystal, Cortés and Herrán use two different metals in nanoscale format. "We first create particles in the range of 10-200 nanometers from a plasmonic metal – which in our case is gold," Herrán explains. "At this scale, a special phenomenon occurs with plasmonic metals, which also include silver, copper, aluminum, and magnesium: visible light interacts very strongly with the electrons of the metal, causing them to oscillate resonantly." This means that the electrons move collectively very quickly from one side of the nanoparticle to the other, creating a kind of mini-magnet. Experts refer to this as a dipole moment. "For the incident light, this is a strong change so that it subsequently interacts much more strongly with the metallic nanoparticle," Cortés explains. "Analogously, one can think of the process as a superlens concentrating the energy. Our nanomaterials do that but on the molecular scale.” This allows the nanoparticles to capture more sunlight and convert it into very high-energy electrons. These, in turn, help drive chemical reactions.
Nano hotspots unleash catalytic power
But how can this energy be harnessed? For that purpose, the LMU scientists teamed up with researchers at the University of Hamburg. They arranged gold particles in an orderly fashion on a surface according to the principle of self-organization. The particles must be very close but not touching for maximized light-matter interactions. In collaboration with a research team from Freie Universität Berlin, which studied the optical properties of the material, the LMU researchers found that light absorption increased many times over. "The gold nanoparticle arrays focus the incoming light extremely efficient, yielding, highly localized and strong electric fields, the so-called hotspots," says Herrán. These form between the gold particles, which gave Cortés and Herrán the idea of placing platinum nanoparticles, a classic and powerful catalyst material, right in the interspaces. This was again done by the research team from Hamburg. "Platinum is not the material of choice for photocatalysis because it absorbs sunlight poorly. However, we can force it in hotspots to enhance this otherwise poor absorption and power chemical reactions with the light energy. In our case, the reaction converts formic acid into hydrogen," Herrán explains. With a hydrogen production rate from formic acid of 139 millimoles per hour and per gram of catalyst, the photocatalytic material currently holds the world record for H2 production with sunlight.
An impetus for greener hydrogen production
Today, hydrogen is primarily produced from fossil fuels, predominantly from natural gas. To switch to a more sustainable production, research teams around the world are working on technologies that use alternative feedstocks – including formic acid, ammonia, and water. The focus is also on developing photocatalytic reactors suitable for large-scale production. "Clever material solutions like ours are an important building block for the success of the technology," mentioned the two researchers. "By combining plasmonic and catalytic metals, we are advancing the development of potent photocatalysts for industrial applications. It is a new way to use sunlight and one that offers potential for other reactions such as the conversion of CO2 into usable substances," Cortés and Herrán explain. The two researchers have already patented their material development.
Read the original article on Ludwig Maximilian University of Munich.