Against the backdrop of climate change and the necessary transition from fossil fuels to renewable energy sources, water electrolysis is becoming increasingly important for the production of hydrogen. However, electrolysis not only requires catalysts for the reduction of protons to hydrogen, but also for the opposite side, namely water oxidation and the associated production of oxygen.
Current processes are extremely energy- and time-intensive and use materials that are difficult to obtain. The development of sustainable, non-toxic and inexpensive - but still active - catalysts is therefore the major current challenge. The novel microwave synthesis presented here enables the synthesis of iron-rich high-entropy transition metal oxides at low temperatures and short times using materials that are among the most common - and therefore most available - elements in the earth's crust.
Currently, electrocatalysts based on iridium or ruthenium oxide are mainly used, which significantly increases material costs and also makes large-scale expansion difficult in terms of material availability. High-entropy transition metal oxides are becoming increasingly interesting for these processes. However, these are usually obtained at high temperatures and with long synthesis times.
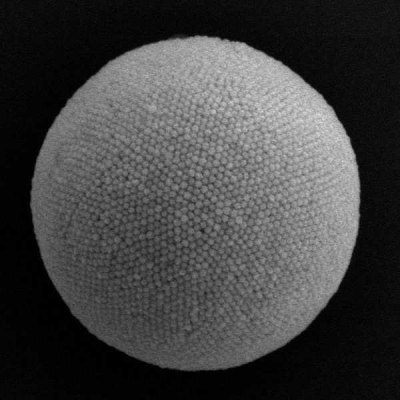
"In this work, we present for the first time a low-temperature synthesis of high-entropy oxides, more precisely of spinels with a high iron content," reports Prof Dr Roland Marschall, holder of the Chair of Physical Chemistry III at the University of Bayreuth. The new type of synthesis in the microwave makes it possible to reduce the synthesis time to minutes (usually 5-30 minutes in this case) and the temperature to 225 °C. On the one hand, the synthesis is therefore much less energy-intensive, and on the other hand, this enables the production of nanoparticles. This is particularly interesting in catalysis, as nanoparticles have a particularly high surface-to-volume ratio and the catalytic reactions required for electrolysis take place on the surface.
"In our work, we were able to show for the first time that a wide variety of different compositions with up to seven different metals in addition to iron can be achieved with this simple low-temperature synthesis," says Prof Marschall. Partially replacing iron with cobalt, which is known for its high activity, enabled an additional increase in catalytic activity. "Finally, the activity of the catalysts depends to a large extent on the composition - but this is not freely variable in all previous synthesis methods. Our method, on the other hand, is highly flexible, which enables the incorporation of a large number of elements in different oxidation states and also allows the composition and thus the activity of the catalysts to be adjusted."
In addition to Prof Marschall, research assistants Dr Judith Zander (supported by BayBatt at the University of Bayreuth and the "SolTech" research network), Julia Petra Wölfel and Dr Morten Weiss were involved in the work. In addition, Dr Yiqun Jiang, Dr Ningyan Cheng and Dr Siyuan Zhang from the Max Planck Institute for Iron Research in Düsseldorf.
Read the original article on University of Bayreuth.