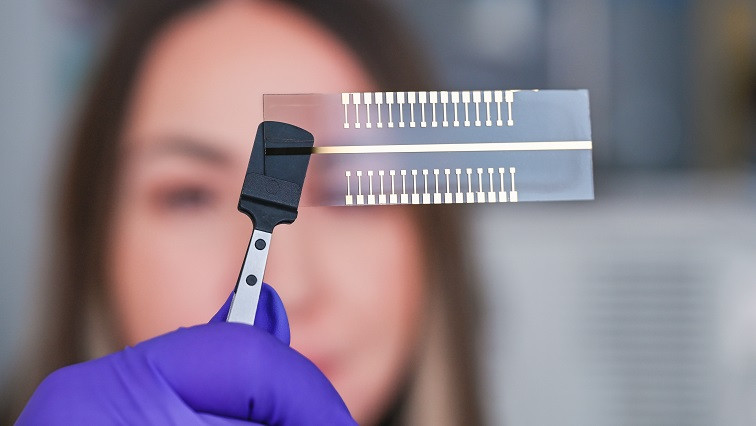
Various possibilities and mechanisms exist for detecting pathogens in body fluids. One option that Baraban investigates at the HZDR-Institute of Radiopharmaceutical Cancer Research is the detection using field-effect transistors (FETs) from the realm of electronics.
The operating principle is simple: a defined electrical current flows from A to B. This current can be regulated by the electrical potential on the surface of a gate, which functions like a precise, continuous valve.
Disease-relevant biomolecules bind to the gate surface and thereby change the electrical potential and therefore the current as well. If there is no significant change in the current, no biomolecules have bound to the sensor surface.
On the other hand, a change in the current means that disease-related molecules can be detected on the sensor surface. These biosensors can be designed to specifically detect different biomolecules. Different pathogens cause different electrical potentials and therefore different currents. Cancer cells cause different current than, for example, a flu virus.
Development of reusable transistors
The major disadvantage of traditional electronic FET-based biosensors is that the test surfaces are not reusable, and the entire transistor must be discarded after each sampling. As transistors contain costly semiconductor materials, this process is both expensive and harmful to the environment.
For that reason, Baraban and her Department of Nano-Microsystems for Life Sciences went one step further and attempted to measure the potential changes not directly on the surface of the transistor, but on a separate electrode that is connected to the transistor’s gate. “This allows us the opportunity to use the transistor multiple times. We separate the gate and refer to it as an ‘extended gate’—that is, an extension of the test system.”
But that’s not all. The team thought even further ahead and took on another challenge: “We, of course, would like this system to carry out several analyses at the same time.” The researchers succeeded in developing extended gates with thirty-two test pads. Baraban explains, “This means that a sample can be tested simultaneously on each of the pads for a different pathogen.”
The scientists first demonstrated the operating principle using interleukin-6 (IL-6), a molecule responsible for communication between immune cells. “Whether it’s a simple cold or cancer, the concentration of IL-6 changes. Different diseases as well as different stages of a disease produce different clinical pictures. That is why IL-6 is very well suited as a marker.”
Nanoparticles to increase sensitivity
In order to make the method even more sensitive, Baraban’s team also utilized nanostructures. Nanoparticles concentrate or localize the charge to amplify the voltage signal. “The sensitivity of the tests is considerably higher than when we work without nanoparticles.”
As ready-made nanoparticle kits for research are now available on the market, this method is simple to use. The HZDR scientists are currently working with gold nanoparticles. In the future, they would also like to study other nanoparticles.
As a result of the current research, a functional, handy test system has been created, consisting of a transistor and thirty-two test pads, with which different pathogens can be detected in a very short period of time. In the future, the described test system could, for example, be used to monitor the progress of immunotherapies in cancer patients.
Another possibility would be to predict the severity and course of a viral disease such as the flu or COVID-19 at the very onset. In comparison to existing technologies, the new system is more cost-effective and faster. For that reason, Baraban and her team are now hoping for interest from the commercial sector.
The team’s current publication in Biosensors and Bioelectronics describes the development of a portable, palm-sized test system that can simultaneously carry out up to thirty-two analyses of one sample.
Read the original article on Helmholtz-Zentrum Dresden-Rossendorf.