Evaporation is a natural process so ubiquitous that most of us take it for granted. In fact, roughly half of the solar energy that reaches the earth drives evaporative processes. Since 2017, researchers have been working to harness the energy potential of evaporation via the hydrovoltaic (HV) effect, which allows electricity to be harvested when fluid is passed over the charged surface of a nanoscale device. Evaporation establishes a continuous flow within nanochannels inside these devices, which act as passive pumping mechanisms. This effect is also seen in the microcapillaries of plants, where water transport occurs thanks to a combination of capillary pressure and natural evaporation.
Although hydrovoltaic devices currently exist, there is very little functional understanding of the conditions and physical phenomena that govern HV energy production at the nanoscale. It’s an information gap that Giulia Tagliabue, head of the Laboratory of Nanoscience for Energy Technology (LNET) in the School of Engineering, and PhD student Tarique Anwar wanted to fill. They leveraged a combination of experiments and multiphysics modelling to characterize fluid flows, ion flows, and electrostatic effects due to solid-liquid interactions, with the goal of optimizing HV devices.
“Thanks to our novel, highly controlled platform, this is the first study that quantifies these hydrovoltaic phenomena by highlighting the significance of various interfacial interactions. But in the process, we also made a major finding: that hydrovoltaic devices can operate over a wide range of salinities, contradicting prior understanding that highly purified water was required for best performance,” says Tagliabue.
The LNET study has recently been published in the Cell Press journal Device.
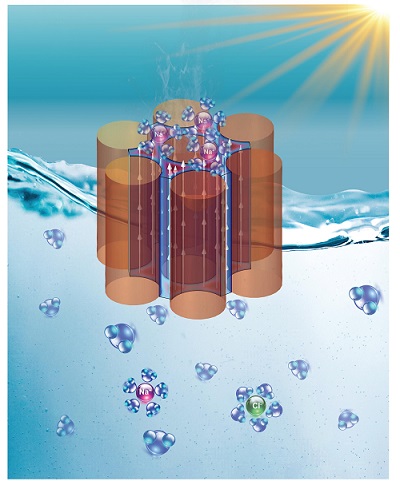
Schematic of an evaporation-driven HV system.
A revealing multiphysics model
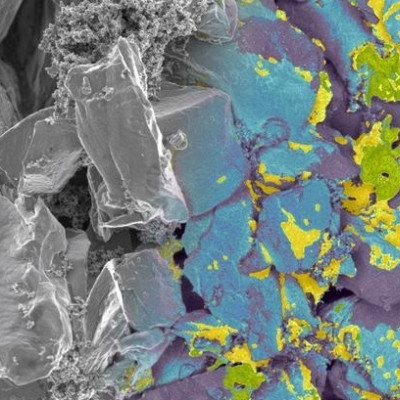
The researchers’ device represents the first hydrovoltaic application of a technique called nanosphere colloidal lithography, which allowed them to create a hexagonal network of precisely spaced silicon nanopillars. The spaces between the nanopillars created the perfect channels for evaporating fluid samples, and could be finely tuned to better understand the effects of fluid confinement and the solid/liquid contact area.
“In most fluidic systems containing saline solutions, you have an equal number of positive and negative ions. However, when you confine the liquid to a nanochannel, only ions with a polarity opposite to that of the surface charge will remain,” Anwar explains. “This means that if you allow liquid to flow through the nanochannel, you will generate current and voltages.”
“This goes back to our major finding that the chemical equilibrium for the surface charge of the nanodevice can be exploited to extend the operation of hydrovoltaic devices across the salinity scale,” adds Tagliabue. “Indeed, as the fluid ion concentration increases, so does the surface charge of the nanodevice. As a result, we can use larger fluid channels while working with higher-concentration fluids. This makes it easier to fabricate devices for use with tap or seawater, as opposed to only purified water.”
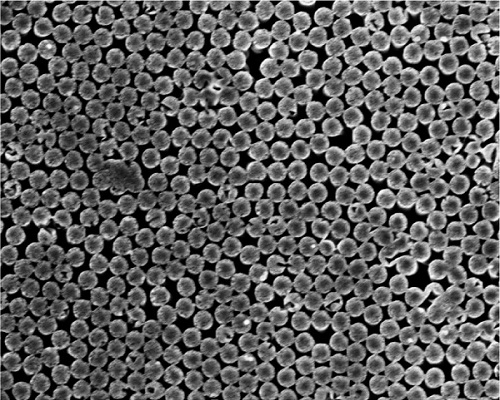
Scanning electron microscope image of the silicon nanopillars.
Water, water everywhere
Because evaporation can occur continuously over a wide range of temperatures and humidities – and even at night – there are many exciting potential applications for more efficient HV devices. The researchers hope to explore this potential with the support of a Swiss National Science Foundation Starting Grant, which aims to develop “a completely new paradigm for waste-heat recovery and renewable energy generation at large and small scales,” including a prototype module under real-world conditions on Lake Geneva.
And because HV devices could theoretically be operated anywhere there is liquid – or even moisture, like sweat – they could also be used to power sensors for connected devices, from smart TVs to health and fitness wearables. With the LNET’s expertise in light energy harvesting and storage systems, Tagliabue is also keen to see how light and photothermal effects could be used to control surface charges and evaporation rates in HV systems.
Finally, the researchers also see important synergies between HV systems and clean water generation.
“Natural evaporation is used to drive desalination processes, as fresh water can be harvested from saltwater by condensing the vapor produced by an evaporative surface. Now, you could imagine using an HV system both to produce clean water and harness electricity at the same time,” Anwar explains.
Read the original article on Ecole Polytechnique Federale de Lausanne (EPFL).