From creating images, generating text, and enabling self-driving cars, the potential uses of artificial intelligence (AI) are vast and transformative. However, all this capability comes at a very high energy cost. For instance, estimates indicate that training OPEN AI's popular GPT-3 model consumed over 1,287 MWh, enough to supply an average U.S. household for 120 years. This energy cost poses a substantial roadblock, particularly for using AI in large-scale applications like health monitoring where large amounts of critical health information are sent to centralized data centers for processing. This not only consumes a lot of energy but also raises concerns about sustainability, bandwidth overload, and communication delays.
Achieving AI-based health monitoring and biological diagnosis requires a standalone sensor that operates independently without the need for constant connection to a central server. At the same time, the sensor must have a low power consumption for prolonged use, should be capable of handling the rapidly changing biological signals for real-time monitoring, be flexible enough to attach comfortably to the human body, and be easy to make and dispose of due to the need for frequent replacements for hygiene reasons.
Considering these criteria, researchers from Tokyo University of Science (TUS) led by Associate Professor Takashi Ikuno have developed a flexible paper-based sensor that operates like the human brain. Their findings were published online in the journal Advanced Electronic Materials on 22 February 2024.
"A paper-based optoelectronic synaptic device composed of nanocellulose and ZnO was developed for realizing physical reservoir computing. This device exhibits synaptic behavior and cognitive tasks at a suitable timescale for health monitoring," says Dr. Ikuno.
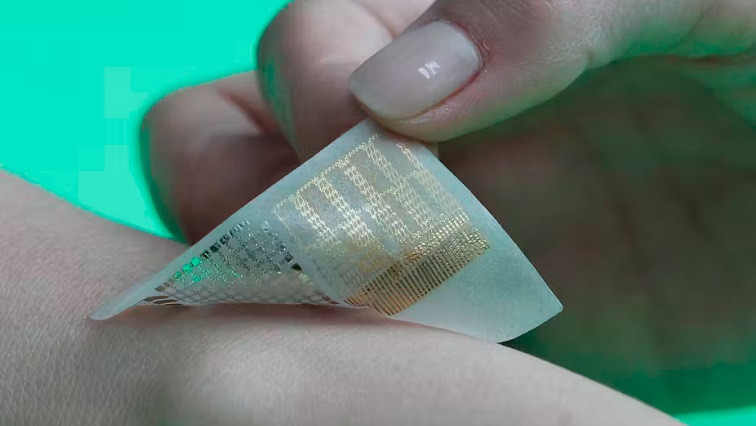
The proposed device is photoresponsive to UV pulses, is flexible, and easy to manufacture and dispose of, making it ideal for health monitoring purposes.
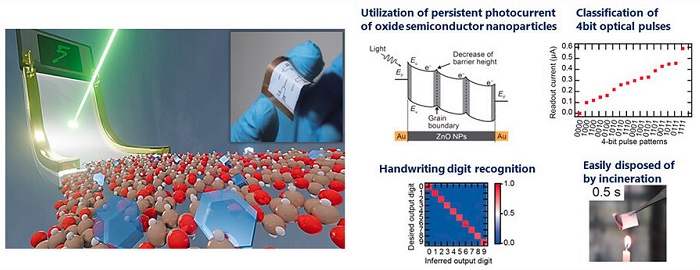
In the human brain, information travels between networks of neurons through synapses. Each neuron can process information on its own, enabling the brain to handle multiple tasks at the same time. This ability for parallel processing makes the brain much more efficient compared to traditional computing systems. To mimic this capability, the researchers fabricated a photo-electronic artificial synapse device composed of gold electrodes on top of a 10 µm transparent film consisting of zinc oxide (ZnO) nanoparticles and cellulose nanofibers (CNFs).
The transparent film serves three main purposes. Firstly, it allows light to pass through, enabling it to handle optical input signals representing various biological information. Secondly, the cellulose nanofibers impart flexibility and can be easily disposed of by incineration. Thirdly, the ZnO nanoparticles are photoresponsive and generate a photocurrent when exposed to pulsed UV light and a constant voltage. This photocurrent mimics the responses transmitted by synapsis in the human brain, enabling the device to interpret and process biological information received from optical sensors.
Notably, the film was able to distinguish 4-bit input optical pulses and generate distinct currents in response to time-series optical input, with a rapid response time on the order of subseconds. This quick response is crucial for detecting sudden changes or abnormalities in health-related signals. Furthermore, when exposed to two successive light pulses, the electrical current response was stronger for the second pulse. This behavior termed post-potentiation facilitation contributes to short-term memory processes in the brain and enhances the ability of synapses to detect and respond to familiar patterns.
To test this, the researchers converted MNIST images, a dataset of handwritten digits, into 4-bit optical pulses. They then irradiated the film with these pulses and measured the current response. Using this data as input, a neural network was able to recognize handwritten numbers with an accuracy of 88%.
Remarkably, this handwritten-digit recognition capability remained unaffected even when the device was repeatedly bent and stretched up to 1,000 times, demonstrating its ruggedness and feasibility for repeated use. "This study highlights the potential of embedding semiconductor nanoparticles in flexible CNF films for use as flexible synaptic devices for PRC," concludes Dr. Ikuno.
Let us hope that these advancements pave the way for wearable sensors in health monitoring applications!
Read the original article on Tokyo University of Science.