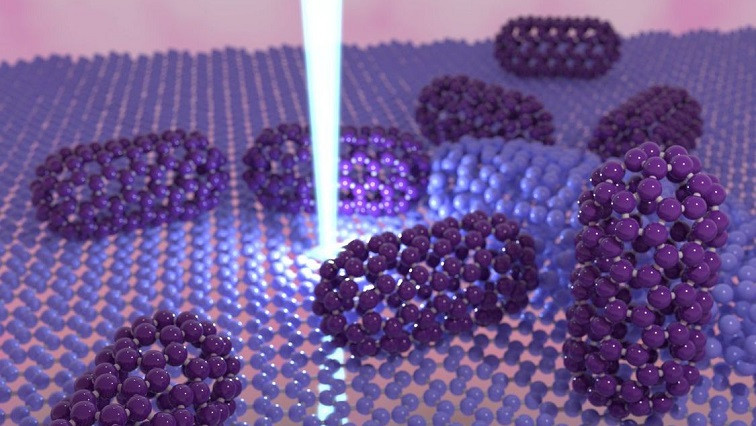
For years, C130 fullertubes—molecules made up of 130 carbon atoms—have existed only in theory. Now, leading an international team of scientists, an UdeM doctoral student in physics has successfully shown them in real life – and even managed to capture some in a photograph.
This feat in the realm of basic research has led Emmanuel Bourret to have a cover-page illustration of his discovery in a prestigious scientific journal, the Journal of the American Chemical Society.
First published online last October, the discovery was made by Bourret as lead scientist of an inter-university team that also included researchers from Purdue University, Virginia Tech and the Oak Ridge National Laboratory, in Tennessee.
A fullertube is basically an assembly of carbon atoms arranged to form a closed tubular cage. It is related to fullerenes, molecules that are represented as cages of interconnected hexagons and pentagons, and come in a wide variety of sizes and shapes.
For example, a C60 fullerene is made up of 60 carbon atoms and is shaped like a soccer ball. It is relatively small, spherical and very abundant. C120 fullerenes are less common. They are longer and shaped like a tube capped at either ends with the two halves of a C60 fullerene.
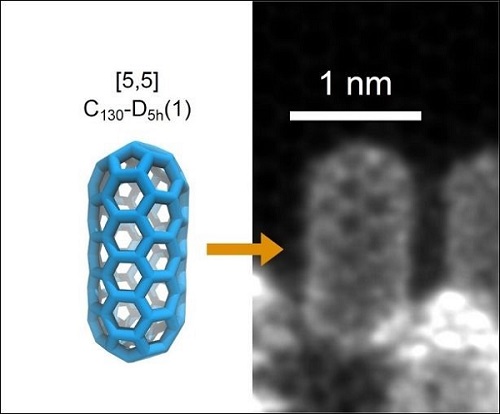
The C130 fullertube measures just under two nanometers long by one nanometer wide (one nanometer is one billionth of a meter!). Its tubular structure is composed of atoms arranged in the shape of a hexagon, while at both ends these hexagons are linked together by pentagons, which gives them their rounded shape.
Found in soot
The C130 fullertube (or C130-D5h, its full scientific name) is more elongated than the C120 and even rarer. To isolate it, Bourret and his team generated an electric arc between two graphite electrodes to produce soot containing fullerene and fullertube molecules. The electronic structure of these molecules was then calculated using density functional theory (DFT).
"Drawing on principles of quantum mechanics, DFT enables us to calculate electronic structures and predict the properties of a molecule using the fundamental rules of physics," explained Bourret's thesis supervisor, UdeM physics professor Michel Côté, a researcher at the university's Institut Courtois.
Using special software, Bourret was able to describe the structure of the C130 molecule: it is a tube with two hemispheres at the ends, making it look like a microscopic capsule. It measures just under 2 nanometres long by 1 nm wide (a nanometre is one billionth of a metre).
"The structure of the tube is basically made up of atoms arranged in hexagons,” said Bourret. “At the two ends, these hexagons are linked by pentagons, giving them their rounded shape."
Bourret began doing theoretical work on fullertubes in 2014 under his then-supervisor Jiri Patera, an UdeM mathematics professor. After Patera passed away in January 2022, Bourret then approached Côté, who became his new supervisor.

One of the C130 fullertubes brought to light by Emmanuel Bourret.
Existence shown in 2020
Two years before that, Bourret had read an article by Purdue University at Fort Wayne professor Steven Stevenson, who described the experimental isolation of certain fullertubes, demonstrating their existence but not identifying all of them.
Under Côté's guidance, Bourret set to work advancing knowledge on the topic.
"Emmanuel had a strong background in abstract mathematics," Bourret recalled, "and he added an interesting dimension to my research group, which focuses on more computational approaches."
Are any possible future applications in the offing?
"It's hard to say at this stage, but one possibility might be the production of hydrogen,” said Côté. “Currently, what's used is a catalyst made of platinum and rubidium, both of which are rare and expensive. Replacing them with carbon structures such as C130 would make it possible to produce hydrogen in a 'greener' way."
Last year, Bourret's groundbreaking work earned him an invitation to deliver a paper at the annual meeting of the U.S. Electrochemical Society (ECS), in Boston. This May, he'll chair a panel on fullerenes and fullertubes at the ECS annual meeting in San Francisco.
Read the original article on University of Montreal.