A nanoparticle-based therapy developed by UT Southwestern Medical Center scientists stimulated an immune pathway that eradicated tumors in mouse models of various cancer types. Their findings, published in Science Immunology, offer a new way to potentially harness the power of the body’s immune system against cancer.
“The gold standard of current cancer immunotherapies releases the brakes on the immune defense against tumors. Our strategy steps on the gas to drive immune activation,” said Jinming Gao, Ph.D., Professor in the Harold C. Simmons Comprehensive Cancer Center and in the Departments of Biomedical Engineering, Cell Biology, Otolaryngology – Head & Neck Surgery, and Pharmacology at UT Southwestern. Dr. Gao co-led the study with Jian Wang, Ph.D., and Suxin Li, Ph.D., both former postdoctoral fellows, and Maggie Wang, M.S., a graduate student in the Gao Lab.
In the past couple of decades, researchers at UTSW and elsewhere have learned more about the immune system’s role in fighting cancer. Several anti-tumor immunotherapies based on this work have been approved by the U.S. Food and Drug Administration and are now regularly used to treat some cancer types. However, these therapies largely fall into a category called checkpoint inhibitors, which block proteins that prevent immune cells from attacking cancer cells. Only about 20% to 30% of cancer patients respond to these therapies.
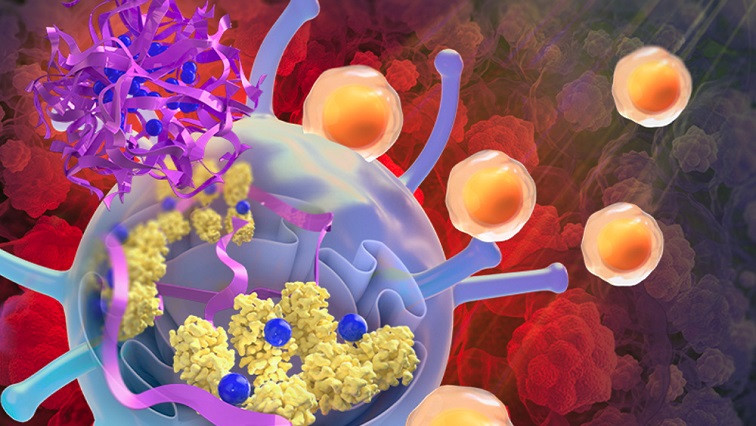
This new nanoparticle-based therapy takes a different approach: activating a molecule known as the stimulator of interferon genes (STING). This evolutionarily ancient molecule responds to a molecular signal called cGAMP. Generated when cells sense an infection or cancer, cGAMP signals immune cells to ready for battle. cGAMP was discovered by UT Southwestern biochemist Zhijian “James” Chen, Ph.D., a world-renowned expert on innate immunity who is Professor of Molecular Biology and in the Center for the Genetics of Host Defense as well as Director of the Center for Inflammation Research.
Activating STING to fight cancer isn’t a new concept, Dr. Gao explained, adding that researchers have been pursuing this goal for several years. However, molecules developed to target STING have largely been ineffective for a variety of reasons, including for draining too quickly from the tumor site or for killing CD8+ T cells in the immune system that attack cancer cells.
In 2017, Dr. Gao and his colleagues discovered that a polymer nanoparticle they designed, called PC7A, activated STING even without stimulation by cGAMP. A follow-up study in 2021 showed that PC7A polymerized STING to continue this activation for over 48 hours, causing a sustained effect.
In the latest study, Dr. Gao’s team created a new experimental therapy that embedded cGAMP in PC7A nanoparticles. The combination initially stimulated STING activation with cGAMP and then locked in this strong activation for an extended period.
Their experiments in mouse models of various cancers showed that this strategy shrank tumors so effectively that it cured some animals of disease, prevented metastasis, or the spread of cancerous tumors, and prevented relapse when cured mice were re-exposed to the same types of cancer cells. Dr. Gao said they learned that the nanoparticle treatment also worked for immunologically “cold” tumor types that usually don’t respond to checkpoint inhibitors.
Searching for the mechanism behind this effect, the researchers found that the nanoparticles preferentially accumulate and act on type 1 conventional dendritic cells, an immune cell type that helps prime CD8+ T cells to fight tumors. Surprisingly, these cells were found to be elevated in cancer patients with longer progression-free survival after treatment with checkpoint inhibitors, Dr. Gao said, confirming that these cells are important for anti-cancer immunity in a clinical setting.
Read the original article on UT Southwestern Medical Center.