In ancient Greece, over 3000 years ago, wise men used silver salts to prevent wounds from becoming infected. These salts continued to be used until Alexander Fleming discovered the first antibiotic “just” 100 years ago. The use of antibiotics represented a major breakthrough in the treatment of infectious diseases, but resistance soon began to emerge. Bacteria, which have been on the planet longer than us, have found ways to overcome different antibiotics, and today antibiotic resistance is a major global health problem.
In times when everything evolves very quickly, it is interesting to gain perspective, to return a bit to the origins. That is why attention has turned back to silver salts, which had so much use years ago and in fact never stopped being used. Silver salts are the basis of microscopic crystals or micromotors constructed by researchers from the Institute of Chemical Research of Catalonia (ICIQ-CERCA) in Tarragona, in collaboration with the Catalan Institute of Nanoscience and Nanotechnology (ICN2). These crystals move autonomously (hence the name micromotors) in aqueous media under visible light irradiation. On their journey, they inactivate present bacteria, becoming a promising tool for environmental recovery.
The group led by Dr. Katherine Villa at ICIQ, in collaboration with ICN2, has published a study in the journal Advanced Optical Materials presenting a simple technique for producing microscopic crystals that activate in the presence of light. Activation involves autonomous movement and the release of silver ions and free radicals with antimicrobial activity, self-degrading, and thus leaving the water free of the crystals themselves.
As Dr. Villa explains, “this work is important because we report a synergistic effect that includes the self-propulsion capability of the micromotors under light stimuli, allowing greater diffusion and dispersion of silver ions as well as released free radicals.”
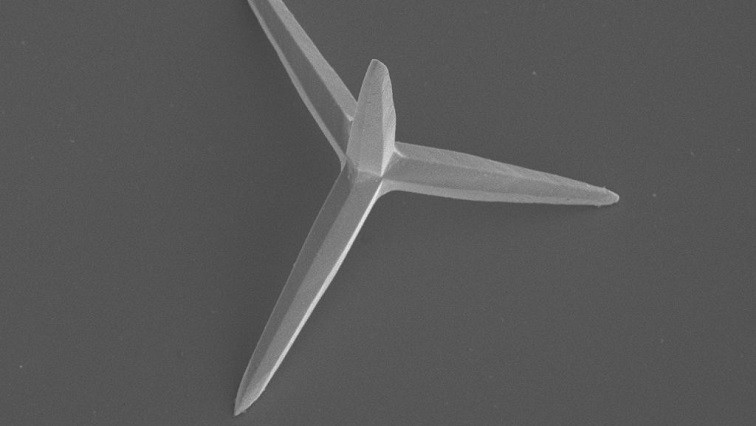
The researchers easily develop microscopic structures containing silver phosphate and shaped like tetrapods. We are not talking about four-legged animals, but a crystalline structure formed by 4 arms, each about 5 micrometers long. These crystals, called TAMs move autonomously through photocatalysis.
We talk about photocatalysis when light acts as a catalyst, in this case, causing the silver phosphate of the TAMs to react with the water in the medium, releasing oxygen, silver ions, and free radicals. The compounds generated from the reaction are responsible for moving the TAMs, and moreover, the released radicals and silver ions kill the bacteria present in the medium. This bactericidal action is explained by the effect of silver on the bacterial walls, affecting their permeability and thus causing irreparable damage to the cell wall, leading the bacteria to death.
The silver ions released from these micromotors become silver nanoparticles that can be easily recovered by filtration, avoiding additional contamination. As Dr. Villa explains, “the micromotors are twice as efficient compared to silver nanoparticles alone, according to the results obtained in the study. Additionally, if we prevent their movement, the antibacterial capacity of these micromotors is drastically reduced.”
Micromotors are a very interesting tool for environmental recovery. Last year, Dr. Villa’s team developed micromotors coated with laccase, a chemical compound that accelerates the conversion of urea into ammonia. Urea is an emerging contaminant, as it is a common product of residential activities (urea is the main component of urine) and various industrial processes, while ammonia is gaining importance as a green energy source; this compound can be decomposed for hydrogen production and can be stored as green fuel.
Read the original article on Institute of Chemical Research of Catalonia (ICIQ).