Patients are still at risk of picking up an infection during their stay at a hospital. This is a serious problem because infections are associated with complications during the healing process or even increase the mortality rate.
A distinction must be made between the body's bacteria and foreign pathogens. The body's bacteria (microbiome) can find gaps in the body's defence mechanism through invasive measures such as catheters and surgical incisions and trigger infections. On the other hand, foreign, multi-resistant bacteria and viruses lurk on door handles and washbasins and are mainly transmitted through hand contact.
The inventors Robert Grass, Lara Pfuderer and Wendelin Stark found a way to uncover the transmission paths of foreign pathogens. The sources of infections are identified, and transmission paths are effectively blocked.
The solution consists of synthetic nanoparticles which mimic the properties of pathogens. The particles are similar in size and spread with similar ease. In contrast to their biological counterparts, they are absolutely harmless. For test purposes, the artificial particles are applied in
the immediate vicinity of the patients and then tracked by taking swipe samples from surfaces.
"We encapsulate DNA-tracers inside the nanoparticles. The tracers work like bar codes which we can read out and, thus, detect individual sources and how they spread," says Lara Pfuderer.
The synthetic nanoparticles have a protective shell and are filled with a tracer material. The tracer consists of a synthetic DNA sequence and, like a barcode, enables unique identification. The inventors use copies of short DNA sequences from fruits, which are safe. The DNA tracers are easily detectable. A swipe with a swab is sufficient to pick up even the smallest amount of DNA from a surface and detect it in a PCR device.
During the pandemic PCR-devices were used for Covid testing and are now ubiquitous in medical facilities. Hospitals have therefore the analytic equipment for monitoring the tracers in their own laboratories. When different tracers are used, multiple test campaigns can run in parallel without interfering with each other. So, multiple pathogen sources can be tracked in parallel or campaigns can be staggered over time.
"Our nanoparticles are remarkable in their resemblance to real pathogens regarding the transmission paths and their susceptibility to disinfectants—without being harmful," says Robert Grass.
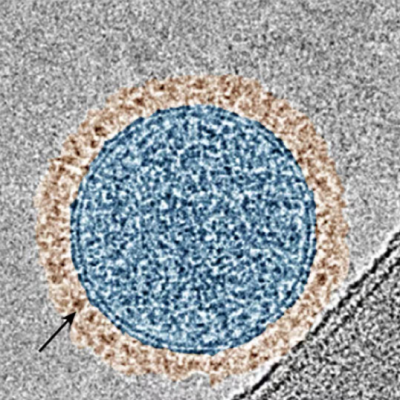
The inventor team developed two variants of the nanoparticles. One variant has a protective shell from silica. This is a robust material, which withstands even disinfectants. This particle variant helps detect the source and its traces until they are lost by dilution.
The second variant of the nanoparticles has a protective shell from lipids and a sugar-glycerin coating (see fig. 2). The lipid-based nanoparticles react like real pathogens to disinfectants. This means a disinfectant that kills a pathogen chemically, will also destroy the nanoparticle. More specifically, the protective shell of the lipid-based nanoparticles breaks open.
The researchers want to quantify how effective the use of disinfectants is. They take a swipe sample and analyse the ratio of released tracers from broken nanoparticles to enclosed tracers from intact nanoparticles. If the quotient is high, a transmission path has been successfully stopped by disinfection.
The inventor team has already launched a few pilot projects in hospitals. They are looking for further opportunities to test their technology in real-life scenarios. Please contact the team.
"For years I have been looking for suitable tracers for the study of transmission pathways. Luckily, I came across Robert Grass and his team. We were able to use their nanoparticles for the prevention of infections in the hospital for the first time. I am convinced that interdisciplinary research and development such as ours leads faster to better solutions," says Hugo Sax.
Read the original article on ETH Zurich.