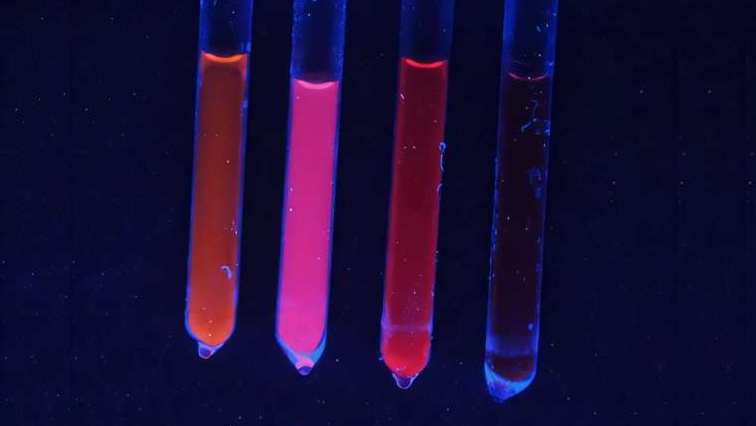
The type of semiconductive nanocrystals known as quantum dots are both expanding the forefront of pure science and also hard at work in practical applications including lasers, quantum QLED televisions and displays, solar cells, medical devices, and other electronics.
A new technique for growing these microscopic crystals, published this week in Science, has not only found a new, more efficient way to build a useful type of quantum dot, but also opened up a whole group of novel chemical materials for future researchers’ exploration.
“I am excited to see how researchers across the globe can harness this technique to prepare previously unimaginable nanocrystals,” said first author Justin Ondry, a former postdoctoral researcher in UChicago’s Talapin Lab.
The team—which included researchers from the University of Chicago, University of California Berkeley, Northwestern University, the University of Colorado Boulder, and Argonne National Laboratory—achieved these remarkable results by replacing the organic solvents typically used to create nanocrystals with molten salt—literally superheated sodium chloride of the type sprinkled on baked potatoes.
“Sodium chloride is not a liquid in your mind, but assume you heat it to such a crazy temperature that it becomes a liquid. It looks like liquid. It has similar viscosity as water. It’s colorless. The only problem was that nobody ever considered these liquids as media for colloidal synthesis,” said Prof. Dmitri Talapin at the UChicago Pritzker School of Molecular Engineering (UChicago PME) and the Chemistry Department.
Why salt?
Quantum dots are among the more well-known nanocrystals, not only for their wide commercial uses but for the recent 2023 Nobel Prize in Chemistry given to the team that discovered them.
“If there is a material from the world of nano that has had an impact on the society in terms of applications, it’s the quantum dot,” said UC Berkeley Prof. Eran Rabani, a co-author of the paper.
However, much of the previous research on quantum dots, including the Nobel work, was around dots grown using combinations of elements from the second and sixth groups on the periodic table, Rabani said. These are called “II-VI” (two-six) materials.
More promising materials for quantum dots can be found elsewhere on the periodic table.
Materials found in the third and fifth groups of the periodic table (III-V materials) are used in the most efficient solar cells, brightest LEDs, most powerful semiconductor lasers, and fastest electronic devices. They would potentially make great quantum dots, but, with few exceptions, it was impossible to use them to grow nanocrystals in solution. The temperatures required to make these materials were too high for any known organic solvent.
Molten salt can handle the heat, making these previously inaccessible materials accessible.
“This distinct advance of molten salt synthesis that Prof. Talapin's group has pioneered for the first time many materials for which previously colloidal synthesis was simply unavailable,” said co-author Richard D. Schaller, who has a joint appointment with Argonne National Laboratory and Northwestern University. “Fundamental as well as applied advances can now be made by with many of these newly available materials and at the same time there is now a whole new synthetic frontier available to the community.”
The Quantum Age
One of the reasons researchers synthesizing nanocrystals overlooked molten salt was because of its strong polarity, said UChicago graduate student Zirui Zhou, second author of the new paper.
Salt’s positively charged ions and negatively charged ions have a strong pull toward each other. Small things like nanocrystals have small surface charges, so researchers assumed the charge would be too weak to push back as salt’s ions pull in. Any growing crystals would be crushed before they could form a stable material.
Or so previous researchers thought.
“It's a surprising observation,” Zhou said. “This is very contradictory to what scientists traditionally think about these systems.”
The new technique can mean new building blocks for better, faster quantum and classical computers, but for many on the research team, the truly exciting part is opening up new materials for study.
“Many eras in human history are defined by the materials humanity had available—think ‘Bronze Age’ or ‘Iron Age,’” Ondry said. “In this work we have unlocked the ability to synthesize nearly a dozen new nanocrystal compositions which will enable future technologies.”
Read the original article on University of Chicago.