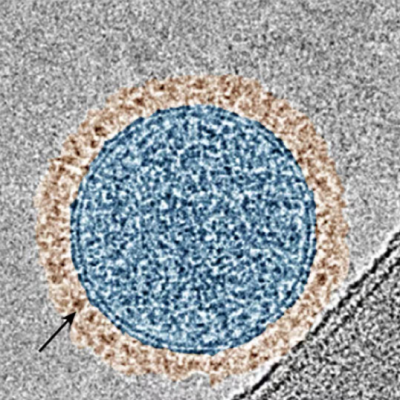
Ateam of scientists and engineers at Sylvester Comprehensive Cancer Center, part of University of Miami Miller School of Medicine, and the UM College of Engineering, in collaboration with the Moffitt Cancer Center and Cellular Nanomed, has developed a first-of-its-kind, minimally invasive approach to treating pancreatic cancer using magnetoelectric nanoparticles (MENPs). This technology was originally created in the lab of UM professor Sakhrat Khizroev in collaboration with Ping Liang of Cellular Nanomed.
The study, published in Advanced Science, demonstrates how MENPs can be guided by magnetic fields to target pancreatic tumors and activated remotely to destroy cancer cells while providing real-time imaging.
“This study brings us one step closer to connecting to the human body wirelessly to help it heal in real time,” said Khizroev, senior author. “We hope it opens a new era in medicine where technology can precisely target diseases that were once considered untreatable.”
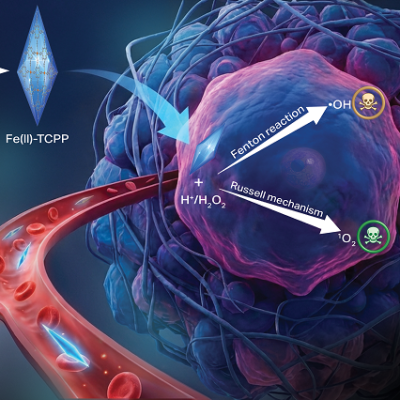
The research shows how MENPs can be delivered directly to pancreatic tumors, where they are remotely activated by a magnetic field inside an MRI scanner. Once activated, the nanoparticles generate local electric fields that distinguish between healthy and cancerous cells based on their molecular properties, causing only the malignant cells to undergo apoptosis, or programmed cell death.
“Magnetoelectric nanotherapy brings a new dimension to theranostic oncology by coupling imaging and controlled physical mechanisms of tumor treatment in real time,” said John Michael Bryant, M.D., first author of the study, Sylvester researcher, and clinical assistant professor in the Department of Radiation Oncology at the Miller School. Dr. Bryant is a doctoral student in Khizroev’s lab. “Positioned at the intersection of engineering, physics, and medicine, it offers a path toward safer, more adaptive and personalized cancer care.”
MRI scans confirmed that this treatment reduced tumor size and produced clear imaging signals, supporting MENPs as a powerful therapy and diagnostic, or “theranostic,” tool. Because the particles function without pharmaceutical drugs or biological reagents, the approach minimizes side effects and could eventually be applied to other difficult-to-treat diseases.
Tackling one of the toughest cancers
Despite major advances in oncology, pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers, with a five-year survival rate below 10%. It is projected to become the second leading cause of cancer-related death in the United States by 2030. Traditional methods, including surgery, radiation, and chemotherapy, often harm healthy tissue, while newer approaches such as immunotherapy have shown limited success.
One of the greatest challenges in treating PDAC lies in controlling the electric fields that influence cancer cell growth. Because human tissue conducts electricity, it has been nearly impossible to manipulate these fields precisely inside the body.
While the current research was conducted in preclinical models, the team believes the findings pave the way for future clinical trials and a new generation of wireless nanomedicine.
From concept to collaboration
The idea of using MENPs to wirelessly control local electric fields was first proposed by Khizroev and Liang in 2011. Over the past decade, the concept evolved through global research partnerships and technological breakthroughs, culminating in this study.
Read the original article on University of Miami.