Adjusting the size and chemistry of nanocrystals within an ultrathin surface can speed up light-driven chemical reactions, according to a University of Michigan Engineering study published in the Journal of the American Chemical Society. The new method works by matching the crystals’ electronic rhythm to the internal vibrations of target molecules.
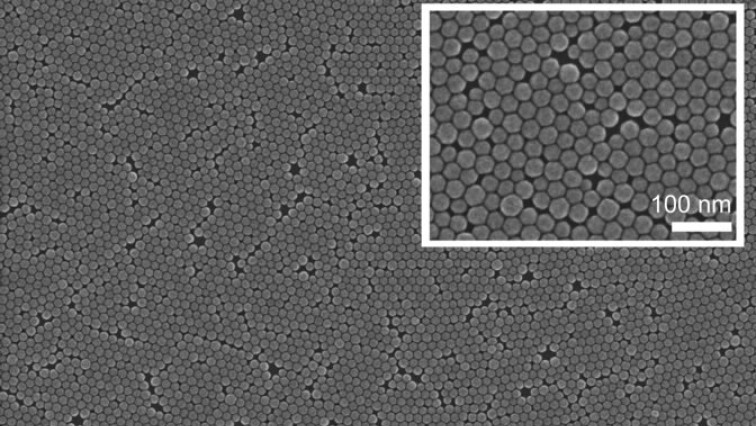
To develop the experimental platform, the research team first synthesized tin-doped indium oxide nanocrystals of a uniform size. The nanocrystals were then assembled into a single, compact layer to create an open metasurface, which is a one-nanocrystal-thick sheet able to manipulate light.
The researchers attached a target molecule directly to the nanocrystal surface and exposed it to a UV light to trigger the reaction. When light interacts with the material, the plasmons are activated within the nanocrystals, which are quantum packets of electron motion.
Monitoring molecular vibrations with a technique called infrared spectroscopy revealed that when the plasmon frequency aligns with the natural vibrational modes of nearby molecules, the electromagnetic field around the nanocrystals can interact directly with specific molecular bonds. This interaction, known as plasmon-vibrational coupling, enables energy exchange between the material and reacting molecules.
By adjusting the nanocrystal composition, the plasmon resonance can be systematically tuned. This allows the rate of the same photochemical reaction to increase or decrease without changing the reactants or reaction conditions.
“Precise tuning of optical resonance frequencies and the concentration of energy in nanoscale volumes is made possible by the chemical synthesis and assembly of metal oxide nanocrystals, which helps us pin down the influence of resonant coupling on chemical reactions,” said Delia Milliron, the Anthony C. Lembke Department Chair of Chemical Engineering and Professor of Chemistry at U-M and co-corresponding author of the study.
Theoretical models validated the experiments, showing that coupling to vibrations when the molecules are excited by light was responsible for speeding up the breaking of chemical bonds.
The results demonstrate that plasmon-vibrational coupling can serve as a controllable parameter for influencing photochemical reaction rates. Rather than modifying the chemistry itself, reaction behavior is shaped by adjusting the optical properties of the surrounding nanomaterial environment.
By isolating how plasmon-vibrational coupling affects reaction kinetics, the work clarifies how energy flows between light, nanomaterials and molecules at the nanoscale. These insights provide a foundation for future studies that explore how similar principles might be applied in more complex chemical systems.
Together, the findings highlight how carefully designed nanomaterials can shape light-matter interactions in ways that influence chemical behavior. As understanding of plasmon-vibrational interactions continues to grow, this research may help inform future methods in areas such as photocatalysis, energy-related chemical transformations and molecular sensing, where precise control over reaction behavior is important.
Read the original article on University of Michigan.