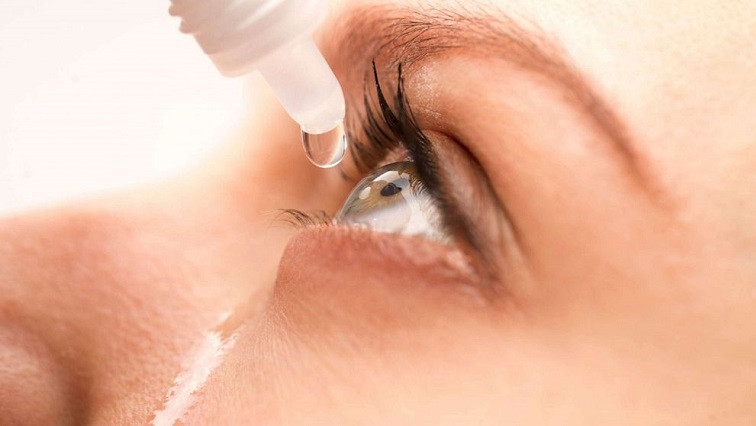
Costas, Inc. DBA Atlas Nanotech, is currently in discussions to license their proprietary nanotechnology vitamin a (retinol) based crystalline eye drops known under the trade name “NANO CLEAR A”, to the leading eye drop manufactures, by licensing Nano Clear A to existing top tier eye drop manufactures it will allow the product to be widely used at a rapid pace.
Atlas Nanotech’s business model is to develop and patent unique nanotechnology products. These unique proprietary products are then licensed to leading manufactures to enhance the performance of existing product offerings. The Company’s scientific team is confident in its ability to license existing products and continue to develop additional nanotechnology products that the company will license to some of the world’s leading biotechnology, pharmaceutical and consumer products manufacturers.
The Company is dedicated to offering their proprietary “NANO CLEAR A” to all industry leaders in the eyedrop and lubricants market. This approach will allow each licensee to brand and market this revolutionary nanotechnology to fit within their existing brands and product offerings. Industry leaders in the global eye drops and lubricants market include Novartis International AG (Alcon, Inc.), Akorn Consumer Health (Thera Tears), Johnson & Johnson (Visine), Pfizer, Similasan Corporation USA, Sager Pharma Kft., Prestige Consumer Healthcare, Inc., Allergan PLC. and Valeant Pharmaceuticals International, Inc. (Bausch & Lamb, Inc.) just to name a few. The Company is currently establishing communications with a number of industry leaders in this market.
The development to be known as “Nano Clear A” represents the culmination of research initiated by Dr. Aldo Oregon Miranda an ophthalmologist surgeon and member of the scientific advisory board of Atlas Nanotech, in collaboration with Professor David De La Mora Atlas’ chairman.
The global eye drops and lubricants market size was valued at $1.8 billion for 2018 and is projected to register substantial growth through 2025. The growth within this segment will be driven by the aging demographics across the globe and the prevalence of ophthalmic disorders worldwide. Management is confident with strategic licensees’ NANO CLEAR A will become the standard in the industry driving top line revenue at a rapid pace.
Read the original article on GlobeNewswire.