Chloroquine and its derivatives have a long history as safe and inexpensive drugs for the prevention of malaria and the treatment of some autoimmune diseases. Cell culture studies have proved that chloroquine has antiviral activity against human coronavirus OC43 and SARS-CoV; its antiviral mechanism, however, needs to be studied further, but based on current evidence, it is hypothesized that the mechanism involves inhibition of viral fusion and prevention of host receptor protein glycosylation as well as viral envelope glycoprotein; or inhibition of virion assembly in the endoplasmic reticulum.
Nanomedicine research on chloroquine and the study of nanoparticle uptake in cells can produce an illuminating insight as to how this medication affects viruses. Accordingly, this anti-malaria drug has been demonstrated to be an effective inhibitor of nanoparticle endocytosis by resident macrophages, thereby decreasing the accumulation of the nanoparticles of various sizes (14 to 2,600 nm) and shapes in cells. Furthermore, it has been proved it reduces the expression of phosphatidylinositol binding clathrin assembly protein (PICALM), which plays a pivotal role in regulating the rate of endocytosis. The deficiency of PICALM leads to the inhibition of clathrin-mediated endocytosis, a predominant pathway for the internalization of the nanoparticles.
Chloroquine is a weak base that can enter membrane-enclosed low-pH organelles and increase their pH, and the pH changes in lysosomes and endocytic vesicles affect their fusion with the cell membrane and interfere with their traffic into and out of the cells. SARS-CoV-2 is spherical with a diameter around 140 nm; therefore, it may enter the cells through clathrin-mediated endocytosis, which can be hindered by chloroquine due to PICALM suppression.
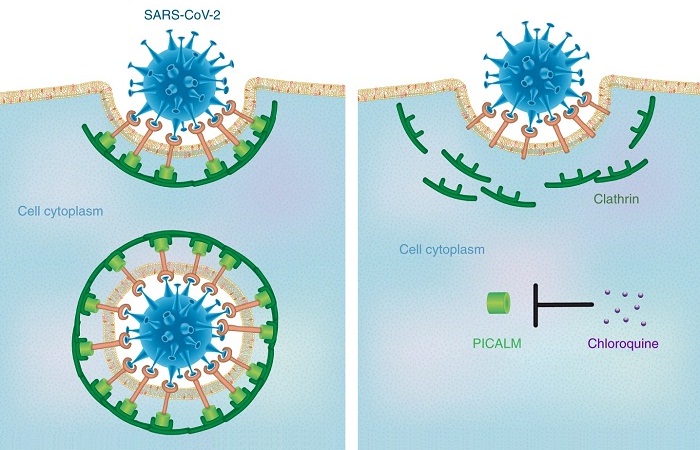
Figure 1. Schematic illustration of chloroquine’s therapeutic mechanism against COVID-19.
Moreover, chloroquine-induced prevention of endosome–lysosome fusion is likely to interfere with general endocytic trafficking, such as membrane receptor recycling, which is thought to be required for SARS-CoV-2 cellular entry.
For entering the cells, SARS-CoV-2 needs the spike protein on the surface of the virion to be cleaved by resident endosomal proteases – such as cathepsins – and undergo conformational changes. Cathepsins are activated upon the acidification of the endosome; thus, chloroquine increasing the pH of the endosome can disturb the process and prevent the virus from entering the cells.
Be that as it may, at this point we cannot make bottom-line claims about the efficacy of chloroquine in the treatment of COVID-19 prior to the receipt of positive clinical trial results, and further studies are also required to achieve the optimal therapeutic clinical protocols regarding patient population, disease stage, and dosing of the drug candidate.
Read the original article on Nature Nanotechnology.