The European project CoNVaT, led from the Catalan Institute of Nanoscience and Nanotechnology (ICN2) by Prof. Laura M. Lechuga (CSIC), will design a new system for the diagnosis of COVID-19, which includes the best of the different existing techniques. Through a biosensor platform based on optical nanotechhnology, it aims to provide a diagnosis of the disease caused by the coronavirus in an accurate and fast manner, and without the need for complex instrumentation.
The University of Barcelona (UB), the University of Aix-Marseille (AMU, France) and the National Institute of Infectious Diseases (INMI, Italy) are collaborating in this project, which has been funded with more than two million euros by the European Union through a fast track call for proposals against COVID-19, within the Horizon 2020 framework. The project will last for two years, but as it is based on already developed technologies, its leaders expect to have applicable results within a year.
The sensor technology developed by the ICN2 consists of a microchip with interferometric waveguides, which currently offers the highest sensitivity for the diagnosis of clinical biomarkers. These microchips allow the detection and quantification of molecules or viruses in a single step, without the need for prior or subsequent amplification, so that the complete analysis can be performed in less than 30 minutes.
On the one hand, it will monitor the binding of the virus to the sensor in real time by means of specific antibodies anchored to the sensor surface, which will allow a rapid diagnostic response and the quantification of the viral load from samples of nasopharyngeal fluid, saliva, or any other fluid of interest. On the other hand, it will identify the viral RNA by means of complementary DNA probes. This genomic assay does not require PCR amplification processes and would allow several simultaneous tests to be performed on the same chip to distinguish which type of virus is contained in the sample.
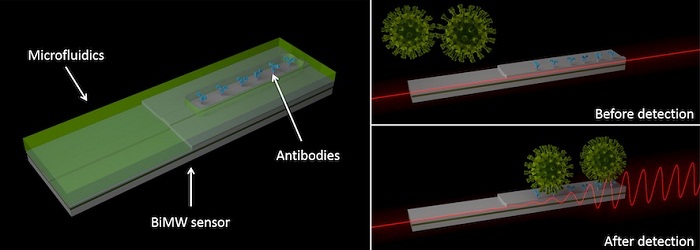
Optical test for Covid-19: Interferometric technology spots Covid-19 virus through changes in optical behavior.
Together with the Hospital de la Vall d'Hebron in Barcelona and the University of Aix-Marseille, the ICN2 has begun to study the possibilities of adapting sensor technology for serological analysis (detection of antibodies in serum).
Today the Spanish Ministry of Science and Innovation has announced that the ICN2 Nanobiosensors and Bioanalytical Applications Group (NanoB2A), led by Prof. Laura M. Lechuga, has prepared a report reviewing the different diagnostic methods available for COVID-19 disease. The new system being developed seeks to surpass some of the features of current tools by taking the best of each strategy.
In general terms, according to the report, the methods for detecting respiratory viruses such as the new coronavirus could be classified into three different strategies:
1- Detection of the genetic material of the virus (RNA)
This is the strategy that uses PCR (Polymerase Chain Reaction). It is a routine technique in clinical laboratories based on the amplification of DNA fragments, so it is necessary to first convert the viral RNA to DNA. Although they are highly specific and sensitive techniques, their main limitation is that they usually require highly specialized personnel, in a process that can take from 2 to 5 hours and is relatively expensive.
There are currently technologies at different stages of development and market reachability that can bring improvements to the weaknesses of this technique. Biosensor devices are notable for their great potential, and could be used in primary and tertiary care centres or even in emergencies, providing high sensitivity and offering real-time measurements.
2- Detection of the virus as an individual entity
In this case the whole virus is detected by the use of specific antibodies directed to interact with the viral antigens. This strategy is the one used by the so-called RADTs (rapid antigen detection tests). This approach is very dependent on the availability of highly specific and quality antibodies. Its speed and low cost is overshadowed by the limited sensitivity and the fact that it does not allow for the quantification of viral load.
Some rapid tests use advanced versions containing reagents (such as nanoparticles) that amplify the signal, improving sensitivity. On the other hand, biosensor devices can again be used for virus detection by immobilizing antibodies specific to coronavirus antigens on the sensor surface.
3- Detection of antibodies in the infected organism (serological test)
The immune system responds to infection by producing antibodies that confer immunity to further reinfection. Serological tests measure the presence of specific antibodies generated in the patient's serum.
These are quick tests that use easily obtained blood samples. They are limited, however, by the need for the immune response to occur (several days after infection), a high probability of false negatives, the lack of quantitative information and the inherent variability in the immune response of each individual.
Biosensor devices, once again, provide greater sensitivity, reproducibility and a quantitative response. This would allow the monitoring of the disease evolution through antibody levels and would provide valuable information to estimate the time of acquired immunity in case of having suffered the COVID-19, a parameter still unknown.
Read the original article on ICN2.







